Právní předpis byl sestaven k datu 27.01.2025.
Zobrazené znění právního předpisu je účinné od 22.08.2023 do 27.01.2025.
32
XXXXXXX
Xxxxxxxxxxxx zahraničních xxxx, xxxxxx se xxxx x doplňují xxxxxxx Xxxxxxxxxxxx zahraničních věcí x. 58/2007 Xx. x. x. x x. 46/2008 Xx. x. s.
Ministerstvo xxxxxxxxxxxx xxxx xxxxxxx, že xxx 15. xxxxxxxxx 2022 bylo xxxxxxxxx xxxxxxxxxx XXXXXX xxxxxxxx xxxxxxxxx novéno xxxxx Xxxxxxx X (Seznam xxxxxxxxxx xxxxx x xxxxx dopingu xxx xxx 2023 - Xxxxxxxxxxx xxxxxxxx) x Xxxxxxx XX - Xxxxxxxxxxx xxxxxxxx xxx xxxxxxxxxxxx xxxxxxx xxx xxx 2023 Mezinárodní xxxxxx proti xxxxxxx xx sportu1).
S novým xxxxxx Xxxxxxx X x Xxxxxxx II xxxxxxxx xxxxxxx Xxxxxxxxx Xxxxx xxxxxxxxx a xxxxxxxxx xxxxxxxxx podepsal xxxxxxx x xxxxxxx xxxx Xxxxxxx X x Xxxxxxx II Xxxxxx republikou.
Nové xxxxx Xxxxxxx X x Xxxxxxx XX vstoupilo x xxxxxxxx x xxxxxxx s článkem 34 xxxx. 3 Xxxxxx xxx 1. xxxxx 2023, pro Českou xxxxxxxxx vstoupilo x xxxxxxxx xxx 22. xxxxxx 2023 x xxxxxxxxx
- Přílohu X xxx xxx 20222), xxxxx vstoupila x xxxxxxxx xxx 1. xxxxx 2022 x xxx Xxxxxx xxxxxxxxx xxxxxxxxx x xxxxxxxx xxx 27. xxxxx 2023, xxxxx xxxxxxxxx Xxxxxxx X xxx xxx 20183), xxxxxxx xx 1. ledna 2018, xxxxx vstoupila x platnost pro Xxxxxx xxxxxxxxx xxx 5. června 2019,
a
- Xxxxxxx XX xxx xxx 20164), která xxxxxxxxx v platnost xxx 14. xxxxxx 2016 a pro Xxxxxx xxxxxxxxx xxxxxxxxx x xxxxxxxx xxx 24. xxxxx 2017.
Xxxxxxxx xxxxx Xxxxxxx X x Xxxxxxx XX xxx xxx 2023 x xxxxxx xxxxxxx xx xxxxxxx jazyka xx xxxxxxxxx současně.
PŘEKLAD
SVĚTOVÝ XXXXXXXXXXXXX XXXXX
XXXXXXXXXXX XXXXXXXX
XXXXXX XXXXXXXXXX
XXXXX X METOD
2023
Tento Xxxxxx je xxxxxx xx 1.1.2023.
XXXXX
Xxxx xxxxxxx&xxxx;Xxxxxx&xxxx;xxxxxxxx xxxxxxxxxxx xxxxx nemusí xxx xxxxx.
|
XXXXX X XXXXXX ZAKÁZANÉ XXXXXX |
|
|
X0 Xxxxxxxxxxx látky |
|
|
S1 Xxxxxxxxxx xxxxx |
|
|
Xxxxxxx z těchto xxxxx xxx bez xxxxxxx xxxxxx x xxxxxx používaných x xxxxx xxxx. xxxxxxxx xxxxxxxxxxxxx. |
|
|
X2 Xxxxxxxxx hormony, xxxxxxx faktory, xxxxxxxx xxxxx x xxxxxxxx |
|
|
Xxxxxxx x xxxxxx xxxxx xxx xxx xxxxxxx xxxxxx x lécích xxxxxxxxxxx x xxxxx xxxx. xxxxxx, mužského xxxxxxxxxxxxx x xxxxxxxxxx xxxxxxxxx xxxxxxx. |
|
|
X3 Beta-2 xxxxxxxx |
|
|
Xxxxxxx z xxxxxx xxxxx xxx bez xxxxxxx nalézt x xxxxxx používaných x xxxxx xxxx. xxxxxxx x xxxxxx respiračních xxxxxx. |
|
|
X4 Xxxxxxxxx a xxxxxxxxxxx xxxxxxxxxx |
|
|
Xxxxxxx x xxxxxx xxxxx xxx xxx xxxxxxx nalézt x xxxxxx xxxxxxxxxxx x xxxxx např. xxxxxxxx xxxx, xxxxxxxx, xxxxxx xxxxxxxxxxx, xxxxxxxx xxxxxxxxxxxxxx xxxxxxxxx. |
|
|
X5 Diuretika x xxxxxxxxx xxxxx. |
|
|
Xxxxxxx x xxxxxx xxxxx xxx bez xxxxxxx xxxxxx x lécích xxxxxxxxxxx k léčbě xxxx. xxxxxxxxx xxxxxxx, xxxxxxxxxx. |
|
|
X1–X2–X3 Xxxxxxxx metody |
|
|
LÁTKY X XXXXXX ZAKÁZANÉ XXX XXXXXXX |
|
|
X6 Stimulancia |
|
|
Některé x xxxxxx xxxxx xxx xxx xxxxxxx xxxxxx x xxxxxx xxxxxxxxxxx x xxxxx xxxx. anafylaxe, xxxxxxx xxxxxxxxxx xxxxx x xxxxxxxxxxxxxx (XXXX), xxxxxxxx xxxxxxxxxx a xxxxxxx. |
|
|
X7 Xxxxxxxxx |
|
|
Xxxxxxx z xxxxxx xxxxx lze xxx xxxxxxx xxxxxx x xxxxxx xxxxxxxxxxx x xxxxx xxxx. xxxxxxx xxxxxx xxxxxxxx pohybového xxxxxxx. |
|
|
X8 Xxxxxxxxxxx |
|
|
X9 Glukokortikoidy |
|
|
Některé x těchto látek xxx xxx xxxxxxx xxxxxx x lécích xxxxxxxxxxx x léčbě xxxx. xxxxxxx, xxxxxxxxx, xxxxxxx, xxxxxxxxxxx xxxxxxxxxxxx xxxxx. |
|
|
XXXXX XXXXXXXX V XXXXXXXXX XXXXXXXX |
|
|
X1 Beta-blokátory |
|
|
Některé x xxxxxx xxxxx xxx xxx omezení xxxxxx x lécích xxxxxxxxxxx x xxxxx xxxx. xxxxxxxxx xxxxxxx, xxxxxxxxxx. |
|
|
XXXXXXXX |
|
XXXXXX XXXXXXXXXX LÁTEK X XXXXX DOPINGU 2023
XXXXXXX ANTIDOPINGOVÝ XXXXX
XXXXXX XX 1. LEDNA 2023
Xxxx
Xxxxxx&xxxx;xxxxxxxxxx xxxxx a xxxxx dopingu (Xxxxxx) xx xxxxxxx&xxxx;Xxxxxxxxxxx xxxxxxxx&xxxx;xxxx xxxxxxx světového xxxxxxxxxxxxxxx xxxxxxxx.
Xxxxxx&xxxx;xx xxxxxxxxxxx jednou xxxxx po xxxxxxxxx xxxxxxxxxxxx xxxxxxx xxxxxxxxxxxxxxxx&xxxx;XXXX. Xxxxx xxxxxxxxx&xxxx;Xxxxxxx&xxxx;xx 1. xxxxx 2023.
Xxxxxxxxx xxxxx&xxxx;Xxxxxxx&xxxx;xxxxxxxxxx xxxxx a xxxxx xxxxxxx je xxx xxxxxxx&xxxx;XXXX&xxxx;x xx publikováno x anglickém x xxxxxxxxxxxx xxxxxx. X xxxxxxx xxxxxxxxxxx konfliktu xxxx xxxxxxxxx x xxxxxxxxxxxx verzí xx xxxxxxxx xx xxxxxxxx.
Xxxx xxxx xxxxxxx xxxxxxx xxxxxxxx xxxxxxxxx x&xxxx;Xxxxxxx xxxxxxxxxx xxxxx a xxxxx.
Xxxxxxxx Při Xxxxxxx
X xxxxxxxx xxxxxx xxxxxx, xxxxx xx&xxxx;XXXX&xxxx;xxxxxxxxx xxx xxxxxx xxxxx, xx xxxxxx&xxxx;Xxx Soutěži v zásadě xxxxxx xxxxxxxxxx xxxxx xxxx xxxxxxx (ve 23:59) xxx xxxx&xxxx;Xxxxxxx, xxxxx se má Sportovec účastnit, xx do xxxxx&xxxx;Xxxxxxx&xxxx;x xxxxxxx odběru Vzorků.
Zakázané xxxxx
Xxxxx xxxx metoda xx xxxxxxxx xxx&xxxx;Xxx Xxxxxxx, xxx x&xxxx;Xxxx Soutěž, xxx xx xxxxxxxxxx x&xxxx;Xxxxxx.
Xxxxxxxxxx a xxxxxxxxxxxx
Xxxxx xxxxxx 4.2.2 Světového xxxxxxxxxxxxxxx Xxxxxx&xxxx;"Xxx xxxxx xxxxxxxx xxxxxx 10 xxxxx xxxxxxx&xxxx;Xxxxxxxx xxxxx&xxxx;Xxxxxxxxxxxx xxxxxxx, xxxxx látek označených x&xxxx;Xxxxxxx. Xxxxx&xxxx;Xxxxxxxx metoda nebude Specifickou xxxxxxx, pokud xxxx x&xxxx;Xxxxxxx&xxxx;xxxxxxxxx označena jako Specifická xxxxxx.". Xxxxx xxxxxxxxx x článku 4.2.2 xxxxx "Specifické látky a metody uvedené x xxxxxx 4.2.2 xxxxx xxxxxxx xxxxxxxxx xx xxxx důležité xxxx xxxx nebezpečné xxx xxxx dopingové xxxxx xx xxxxxx. Xxxxxxxxx xxx xxxxx x xxxxx, které Sportovec s xxxxx xxxxxxxxxxxxxxxx xxxxxxx xx xxxxx xxxxxx xxx xxx zvýšení xxx xxxxxxxxx xxxxxxxxxx."
Xxxxxxxx xxxxx
Xxxxx xxxxxx 4.2.3&xxxx;Xxxxxx&xxxx;xxxx&xxxx;Xxxxxxxx xxxxx&xxxx;xxxxxxxxxx xxxx "Xxxxxxxx xxxxx, které jsou x&xxxx;Xxxxxxx&xxxx;xxxxxxxxx označeny xxxx&xxxx;Xxxxxxxx xxxxx&xxxx;x xxxxxx jejich xxxxxxx zneužívání ve xxxxxxxxxxx mimo xxxxx xxxxxx". Následující látky xxxx označovány jako Návykové xxxxx: kokain, diamorfin (xxxxxx), xxxxxxxxxxxxxxxxxxxxxxxx (XXXX/"xxxxxx") x xxxxxxxxxxxxxxxxxxx (XXX).
X0 XXXXXXXXXXX XXXXX
XXXXXXXX XXXXX (XXX XXXXXXX X XXXX XXXXXX)
Xxxxxxx xxxxxxxx xxxxx xxxx xxxxxxxxxx xx&xxxx;Xxxxxxxxxx xxxxx.
Xxxxxxxxx xxxxxxxxxxxxx xxxxx, která xxxx xxxxxxxx v následujících xxxxxxxxxxx&xxxx;Xxxxxxx&xxxx;x xxxx aktuálně xxxxxxxxx xxx xxxxxxx xxxxxxxxxxxx xxxxxxx xxxxxxxxxx xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx xxxxxx (např. xxxxxx x xxxxxxxxxxxx xxxx xxxxxxxxx xxxxxx xxxxxxx xxxx xx xxxxxxxxx xxxxxxx, xxxxxxxxxx xxxxx, xxxxx xxxxxxxxx xxxxx xxx xxxxxxxxxxx použití), xx zakázána xxxxx.
Xxxx xxxxxxxxx xxxxxxxx xxxxx xxxxxxx látek včetně, xxx xxx xxxxxxx xx BPC-157.
S1 ANABOLICKÉ XXXXX
XXXXXXXX XXXXX (XXX XXXXXXX I XXXX XXXXXX)
Xxxxxxx zakázané xxxxx x xxxx xxxxxxxxx xxxx xxxxxxxxxx xx Xxxxxxxxxxxx xxxxx.
Xxxxxxxxxx xxxxx xxxx xxxxxxxx.
1.&xxxx;XXXXXXXXXX ANDROGENNÍ XXXXXXXX (XXX)
Xxx xxxxxxxxx xxxxxx včetně, ale xx s omezením xxxxx xx xx:
•&xxxx;1-xxxxxxxxxxxxxx (5α-xxxxxxx-1-xx-3ß,17ß-xxxx)
•&xxxx;1-xxxxxxxxxxxxxx (5α-androst-1-en-3,17-dion)
• 1-androsteron (3α-xxxxxxx-5α-xxxxxxx-1-xx-17-xx)
•&xxxx;1-xxxxxxxxxxxxxx (3ß-xxxxxxx-5α-xxxxxxx-1-xx-17-xx)
•&xxxx;1-xxxxxxxxxxx (17ß-xxxxxxx-5α-xxxxxxx-1-xx-3-xx)
•&xxxx;4-xxxxxxxxxxxxxx (xxxxxxx-4-xx-3ß,17ß-xxxx)
•&xxxx;4-xxxxxxxxxxxxxxxxxx (4,17ß-xxxxxxxxxxxxxxxx-4-xx-3-xx)
•&xxxx;5-xxxxxxxxxxxxxx (androst-5-en-3,17-dion)
• 7α-hydroxy-DHEA
• 7ß-hydroxy-DHEA
• 7-keto-DHEA
• 17α-methylepithiostanol (xxxxxxxx)
•&xxxx;19-xxxxxxxxxxxxxxxxx (xxxx-4-xx-3,17-xxxx)
•&xxxx;19-xxxxxxxxxxxxxxxxx (estr-4-en-3,17-dion)
• Androst-4-ene-3,11,17-trione (11-xxxxxxxxxxxxxxxxxxx, xxxxxxxxxxxxx)
•&xxxx;xxxxxxxxxxxxx (5α-xxxxxxxxxxxxxxxxxx, 17ß-xxxxxxx-5α-xxxxxxxxx-3-xx)
•&xxxx;xxxxxxxxxxxxxx (xxxxxxx-5-xx-3ß,17ß-xxxx)
•&xxxx;xxxxxxxxxxxxxx (xxxxxxx-4-xx-3,17-xxxx)
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxx
•&xxxx;xxxxxxx (androsta-1,4-dien-3,17-dion)
• danazol ([1,2]xxxxxxx[4’,5’:2,3]xxxxxx-4-xx-20-xx-17α-xx)
•&xxxx;xxxxxxxxxxxxxxxxxxxxxxxxxxxxx (4-xxxxxx-17ß-xxxxxxx-17α-xxxxxxxxxxxxx-1,4-xxxx-3-xx)
•&xxxx;xxxxxxxxxxxxxxxxxxxxxx (17α-xxxxx-5α-xxxxxxx-2-xx-17ß-xx x 17α-metyl-5α-androst-3-en-17ß-αl)
• drostanolon
• epiandrosteron (3ß-xxxxxxx-5α-xxxxxxxxx-17-xx)
•&xxxx;xxx-xxxxxxxxxxxxxxxxxx (17ß-xxxxxxx-5ß-xxxxxxxxx-3-xx)
•&xxxx;xxxxxxxxxxxxxx
•&xxxx;xxxxxxxxxxxx (19-xxxxxxxxx-4-xx-17α-xx)
•&xxxx;xxxxxxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxx (17α-xxxxx [1,2,5] xxxxxxxxxx[3’,4,:2,3]-5α-xxxxxxxxx-173-xx)
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxxx (17ß-xxxxxxx-17α-xxxxxxxxxxxxx-1,4-xxxx-3-xx)
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxx (17ß-xxxxxxx-2α,17α-xxxxxxx-5α-xxxxxxxxx-3-xx)
•&xxxx;xxxxx-1-xxxxxxxxxxx (17ß-xxxxxxx-17α-xxxxx-5α-xxxxxxx-1-xx-3-xx)
•&xxxx;xxxxxxxxxxxxxx
•&xxxx;xxxxxxxxxxxxx (17ß-xxxxxxx-17α-xxxxxxxxxx-4,9-xxxx-3-xx)
•&xxxx;xxxxxxxxxxxxxxxxxxx (17ß-xxxxxxx-17α-xxxxxxxxx-4-xx-3-xx)
•&xxxx;xxxxxxxxxxxxxxxx
•&xxxx;xxxxxxxxxx (xxxxxxxxxxxxxx) (17ß-xxxxxxx-17α-xxxxxxxxxx-4,9,11-xxxxx-3-xx)
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxx (19-xxxxxxxxxxxxxx)
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxxxx (4-xxxxxx-17ß-xx-xxxx-4-xx-3-xx)
•&xxxx;xxxxxxxxxxxxxx
•&xxxx;xxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxx (dehydroepiandrosteron, XXXX, 3ß-xxxxxxxxxxxxxx-5-xx-17-xx)
•&xxxx;xxxxxxxxxxx (17ß-[(tetrahydropyran-2-yl)oxy]-1’H-pyrazolo[3,4:2,3]-5α-androstan)
• quinbolon
• stanozolol
• stenbolon
• testosteron
• tetrahydrogestrinon (17-xxxxxxx-18x-xxxx-19-xxx-17α-xxxxxx-4,9,11-xxxxx-3-xx)
•&xxxx;xxxxxxx
•&xxxx;xxxxxxxxx (17ß-xxxxxxxxxxx-4,9,11-xxxxx-3-xx)
x další xxxxx x xxxxxxxx xxxxxxxxx xxxxxxxxxx nebo xxxxxxxxx biologickými xxxxxx.
2.&xxxx;XXXXXXX XXXXXXXXXX XXXXX
Xxxxxx, xxx xx s xxxxxxxx xxxxx xx xx:
Xxxxxxxxxxx, xxxxxxxxxxxx, xxxxxxxxxx, selektivní xxxxxxxxxx androgenových xxxxxxxxx (XXXX, xxxx. andarin, xxxxxxxxx (ostarin), LGD-4033 (xxxxxxxxx), RAD140, X-23 x XX-11), zeranol x zilpaterol.
S2 PEPTIDOVÉ XXXXXXX XXXXXXX XXXXXXX, XXXXXXXX XXXXX A XXXXXXXX
XXXXXXXX XXXXX (PŘI XXXXXXX X XXXX XXXXXX)
Xxxxxxx zakázané xxxxx x xxxx xxxxxxxxx xxxx považovány za Nespecifické xxxxx.
Xxxxxxxxxxx xxxxx a xxxxx xxxxx x xxxxxxxx xxxxxxxxx strukturou xxxx xxxxxxxxx biologickými xxxxxx xxxx xxxxxxxx.
1.&xxxx;XXXXXXXXXXXXX (XXX) X PROSTŘEDKY XXXXXXXXXXX XXXXXXXXXXX
Xxxxxx, xxx xx s xxxxxxxx xxxxx na xx:
1.1&xxxx;Xxxxxxxx xxxxxxxxxxxxxxxxx xxxxxxxxx, xxxx. xxxxxxxxxxxx (xXXX); erytropoetiny (XXX); xxxxxxxxxx xxxxxxxx xx XXX, xxxx. XXX-Xx, metoxypolyetylenglykol-epoetin xxxx (XXXX); XXX-xxxxxxxxx xxxxxxxxxx x xxxxxx xxxxxxxxxx, xxxx. XXXX-530, peginesatid.
1.2 Aktivační xxxxxxxxxx hypoxii vyvolávajícího xxxxxxx (XXX), např. xxxxxx; xxxxxxxxxxx (XXX1278863); XXX2 molidustat (XXX 85-3934); roxadustat (FG-4592); xxxxxxxxxx (AKB-6548); xenon.
1.3 Inhibitory XXXX, např. X-11706.
1.4&xxxx;Xxxxxxxxxx xxxxxxxxxxx transformujícího růstového xxxxxxx xxxx (XXX-ß), xxxx. xxxxxxxxxxxx; sotatercept.
1.5 Agonisté xxxxxxxxx xxxxxxxxx xxxxxxxxx, xxxx. asialo XXX; xxxxxxxxxxxxx XXX (CEPO).
2. PEPTIDOVÉ XXXXXXX A XXXXXX XXXXXXXXXX XXXXXXX
2.1&xxxx;Xxxxxxxxxxxxxxxxxx (XX) x luteinizační xxxxxx (XX) x jejich xxxxxxxxxx faktory u xxxx, xxxx. xxxxxxxxx, xxxxxxxxxx, gonadorelin, goserelin, xxxxxxxxxxx, nafarelin x xxxxxxxxxxx.
2.2&xxxx;Xxxxxxxxxxxxxx a xxxxxx xxxxxxxxxx xxxxxxx, xxxx. xxxxxxxxxxxx.
2.3&xxxx;Xxxxxxx xxxxxx (XX), xxxx xxxxxxx a xxxxxxxxx, xxxxxx, ale xx x xxxxxxxx xxxxx xx xx:
•&xxxx;xxxxxxx xxxxxxxxx xxxxxxx, xxxx. xxxxxxxxxxxxxxxxx, somapacitan x xxxxxxxxxx;
•&xxxx;xxxxxxxxx xxxxxxxxx xxxxxxx, xxxx. XXX-9604 x xXX 176-191.
2.4&xxxx;Xxxxxxx xxxxxxxxxx xxxxxxx hormon, xxxxxx, xxx xx x xxxxxxxx xxxxx xx xx:
•&xxxx;xxxxxx xxxxxxxxxx xxxxxxx xxxxxx (XXXX) x xxxx xxxxxxx, např. XXX-1293, XXX-1295, xxxxxxxxxx xxx xxxxxxxxxxx);
•&xxxx;xxxxxxxxxxx xxxxxxxxx xxxxxxx (XXX) x xxxxxx xxxxxxxx, např. xxxxxxxxxxx (xxxxxxx), anamorelin, xxxxxxxxxx, macimorelin x xxxxxxxxxxx;
•&xxxx;xxxxxxxxxx xxxxxxx xxxxxxxxx xxxxxxx XX (XXXX), xxxx. xxxxxxxxxxxx, GHRP-1, XXXX-2 (xxxxxxxxxxx), GHRP-3, XXXX-4, XXXX-5, XXXX-6, x xxxxxxxxxx (xxxxxxxxx).
3.&xxxx;XXXXXXX XXXXXXX X XXXXXXXXXX XXXXXXXXX XXXXXXX
Xxxxxx, xxx xx x xxxxxxxx xxxxx xx xx:
•&xxxx;xxxxxxxxxxxxx xxxxxxx faktory (XXX);
•&xxxx;xxxxxxxxxxxx xxxxxxx xxxxxx (XXX);
•&xxxx;xxxxxxxx xxxxxxx xxxxxxx xxxxxx 1 (XXX-1) x xxxx xxxxxxx;
•&xxxx;xxxxxxxxxx růstové xxxxxxx (MGF);
• růstový faktory xxxxxxxx x xxxxxxxx xxxxxxxx (XXXX);
•&xxxx;xxxxxxxx-ß4 x xxxx deriváty, xxxx. XX-500;
•&xxxx;xxxxxxxxxx-xxxxxxxxxxxx xxxxxxx faktor (XXXX);
x xxxxx xxxxxxx xxxxxxx xxxx xxxxxxxxxx xxxxxxxxx xxxxxxx xxxxxxxxxxx xxxxxxx nebo degradaci xxxxxxxx svalů, xxxxx xxxx xxxxxx, xxxxxxxxxxxxx, xxxxxxx xxxxxxx, xxxxxxxxxxxxx xxxxxxxx xxxx xxxxxxxxxxx xxxx xxxxxxxxx xxxxxx.
X3 XXXX-2 AGONISTÉ
ZAKÁZANÉ STÁLE (XXX XXXXXXX I XXXX XXXXXX)
Xxxxxxx zakázané xxxxx v xxxx xxxxxxxxx xxxx xxxxxxxxxx xx&xxxx;Xxxxxxxxxx látky.
Všichni xxxxxxxxxx x xxxxxxxxxxxx xxxx-2 xxxxxxxx xxxxxx všech xxxxxxxxx xxxxxxx jsou xxxxxxxx.
Xxxxxx, xxx xx x xxxxxxxx pouze xx ně:
• arformoterol
• fenoterol
• formoterol
• higenamin
• indakaterol
• levosalbutamol
• olodaterol
• prokaterol
• reproterol
• salbutamol
• salmeterol
• terbutalin
• tretoquinol (xxxxxxxxxxxxx)
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxx
XXXXXXX
•&xxxx;Xxxxxxxxx xxxxxxxxxx: xxxxxxxxx 1600 xxxxxxxxxx za 24 xxxxx v xxxxxxxxxx xxxxxxx, nepřekračujících 600 xxxxxxxxxx během 8 xxxxx po jakékoliv xxxxx;
•&xxxx;xxxxxxxxx xxxxxxxxxx: maximálně xxxxxx xxxxx 54 xxxxxxxxxx za 24 xxxxx;
•&xxxx;xxxxxxxxx xxxxxxxxxx: maximálně 200 xxxxxxxxxx za 24 xxxxx;
•&xxxx;xxxxxxxxx vilanterol: xxxxxxxxx 25 xxxxxxxxxx xx 24 xxxxx.
! XXXXXXXX
Xxxxxxxxxx salbutamolu x xxxx x xxxxxxxxxxx xxxxx xxx 1000 xx/xx x přítomnost xxxxxxxxxxx x moči x xxxxxxxxxxx xxxxx xxx 40 xx/xx xxxxxx xxxxxxxxxx za xxxxxxxxxxxx použití, ale xxxx xxxxxxxxxx xx&xxxx;Xxxxxxxxx xxxxxxxxxxx xxxxx (XXX), xxxxx&xxxx;Xxxxxxxxx&xxxx;xxxxxxxxx xxxxxxxxxxxxx farmakokinetickou xxxxxx, xx abnormální xxxxxxxx byl xxxxxxxx xxxxxxxxxxxxx dávkou (x xxxxxxxx) xxxxx xxx xxxx xxxxxxx maximální xxxxx.
X4 XXXXXXXXX X XXXXXXXXXXX XXXXXXXXXX
XXXXXXXX XXXXX (XXX XXXXXXX I XXXX XXXXXX)
Xxxxxxxx xxxxx&xxxx;x xxxxxxxxxxx X4.1. a X4.2. xxxx považovány xx&xxxx;Xxxxxxxxxx látky. Xxxxx x xxxxxxxxxxx X4.3. x S4.4. xxxx xxxxxxxxxx xx&xxxx;Xxxxxxxxxxxx xxxxx.
Xxxxxxxxxxx xxxxxxxxx x metabolické xxxxxxxxxx jsou xxxxxxxx.
4.1.&xxxx;XXXXXXXXXX XXXXXXXX
Xxxxxx, xxx xx x xxxxxxxx pouze xx ně:
• 2-androstenol (5α-xxxxxxx-2-xx-17-xx)
•&xxxx;2-xxxxxxxxxxx (5α-xxxxxxx-2-xx-17-xx)
•&xxxx;3-xxxxxxxxxxx (5α-androst-3-en-17-ol)
• 3-androstenon (5α-xxxxxxx-3-xx-17-xx)
•&xxxx;4-xxxxxxxxx-3,6,17 xxxxx (6-xxx)
•&xxxx;xxxxxxxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxx-1,4,6-xxxxx-3,17-xxxx (xxxxxxxxxxxxxxxxx)
•&xxxx;xxxxxxxx-3,5-xxxx-7,17-xxxx (xxxxxxxxx)
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxx
•&xxxx;xxxxxxxxxxx
4.2.&xxxx;XXXXXXXXXXXXXX XXXXX (XXXXXXXXXXXXX X XXXXXXXXXX XXXXXXXXXX XXXXXXXXXXXXX XXXXXXXXX (XXXXX))
Xxxxxx, xxx ne x xxxxxxxx pouze xx xx:
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxx
4.3.&xxxx;XXXXX XXXXXXXXXXX AKTIVACI XXXXXXXXX XXXXXXXX XXX
Xxxxxx, xxx ne s xxxxxxxx xxxxx xx xx:
•&xxxx;xxxxxxxxxx xxxxxxxxxxxxxx xxxxxxx X;
•&xxxx;xxxxxxxxxx xxxxxxxxx xxxxxxxx XXX xxxx například:
- falešné xxxxxxxxx aktivinu, xxxx. XXX-031;
•&xxxx;xxxxxxxxxx proti xxxxxxxxx xxxxxxxx XXX, např. xxxxxxxxxx;
•&xxxx;xxxxxxxxxx xxxxxxxxxx jako xxxxxxxxx:
-&xxxx;xxxxx xxxxxxxxxx xxxx xxxxxx xxxxxxx xxxxxxxxxx;
-&xxxx;xxxxxxxx xxxxxx xxxxxxxxx, xxxx. xxxxxxxxxxx, xxxxxxxxxxxxxxxxxx;
-&xxxx;xxxxxxxxxx xxxxxxxxxxxxxx xxxxxxxxx, např. xxxxxxxxxxx, xxxxxxxxxxxx, landogrozumab, xxxxxxxxxx.
4.4.&xxxx;XXXXXXXXXXX XXXXXXXXXX
4.4.1&xxxx;xxxxxxxxxx XXX-xxxxxxxxxx proteinkinázy (XXXX), xxxx. AICAR, XX9009 x xxxxxxxx xxxxxxxxx 5 xxxxxxxxxxxx xxxxxxxxxxxxxx proliferáty (XXXXα), 2-(2-xxxxx-4-((4-xxxxx-2-(4-(xxxxxxxxxxxxxx)xxxxx)xxxxxxx-5-xx)xxxxxxxxx)xxxxxxx) kyselina octová (XX1516, GW501516);
4.4.2 insuliny x xxxxxxxx xxxxxxxx;
4.4.3&xxxx;xxxxxxxxx;
4.4.4&xxxx;xxxxxxxxxxxx.
X5 DIURETIKA X MASKOVACÍ XXXXX
XXXXXXXX XXXXX (PŘI XXXXXXX X MIMO SOUTĚŽ)
Všechny xxxxxxxx látky v xxxx kategorii xxxx xxxxxxxxxx za Specifické xxxxx.
Xxxxxxx xxxxxxxxx x xxxxxxxxx xxxxx, včetně xxxxxx xxxxxxxxxx všech optických xxxxxxx, xxxx.&xxxx;x-&xxxx;x&xxxx;x-, xxxx xxxxxxxx.
Xxxxxx, xxx ne x xxxxxxxx pouze xx ně:
• desmopressin; xxxxxxxxxx; xxxxxxxxxxxxxxx, xxxx. xxxxxxxxxx xxxxxx xxxxxxxx, xxxxxxxx, xxxxxxxxxxx xxxxxx a xxxxxxxxx;
•&xxxx;xxxxxxxxxxxx; xxxxxxxx; xxxxxxxxx; xxxxxxxxx; xxxxxxxxxxxx; xxxxxxxxx; xxxxxxxx; xxxxxxxx xxxxxxxxxx; xxxxxxxxx; spironolakton; xxxxxxxx, xxxx. bendroflumetiazid, xxxxxxxxxxxxxxxxxx x xxxxxxxxxxxxx; torasemid, xxxxxxxxxx x vaptany, xxxx. xxxxxxxxx.
X xxxxx xxxxx x xxxxxxxx xxxxxxxxx xxxxxxxxxx nebo xxxxxxxxx biologickými xxxxxx.
XXXXXXX
•&xxxx;xxxxxxxxxxx; xxxxxxxx x xxxxxxx xxxx xxxxxx karboanhydrázy, xxxx. xxxxxxxxxxx, xxxxxxxxxxxx);
•&xxxx;xxxxxxx xxxxxx felypressinu xxx xxxxx xxxxxxxxx.
! POZNÁMKA
Nález xxxxxxxxxxx xxxxxxxx xxxxx xx stanoveným xxxxxxxx xxxxxxx, xx. u xxxxxxxxxxx, xxxxxxxxxxx, xxxxxx, xxxxxxxx, xxxxxxxxxxxxx x xxxxxxxxxxxxxx xx&xxxx;Xxxxxx Xxxxxxxxx&xxxx;xxxxxxxx xxxx případně Při Xxxxxxx&xxxx;xx xxxxxxx x xxxxxxxxxx xxxx xxxxx xxxxxxxxx xxxxxx (kromě xxxxxxxxx xxxxxx xxxxxx xxxxxxxxxx xxxxxxxxxxxxxx xxxx lokálního xxxxxx felypresinu x xxxxxxxx xxxxxxxxx), bude xxxxxxxxx xx&xxxx;Xxxxxxxxx xxxxxxxxxxx xxxxx (AAF), pokud Sportovec nemá xxxxxxxxxx&xxxx;Xxxxxxxxxxxxx xxxxxxx (XX)&xxxx;xx xxxx xxxxx navíc x xx, xxxxx xxx xxxx udělena xx diuretikum xxxx xxxxx xxxxxxxxx xxxxx.
XXXXXXXX XXXXXX
XXXXXXXX XXXXX (XXX XXXXXXX X XXXX XXXXXX)
Xxxxxxx xxxxxxxx xxxxxx x xxxx xxxxxxxxx xxxx xxxxxxxxxx xx&xxxx;Xxxxxxxxxxxx xxxxxx, xxxxx metod x X2.2., xxx xxxx&xxxx;Xxxxxxxxxx xxxxxx.
X1.&xxxx;XXXXXXXXXX S XXXX X KREVNÍMI XXXXXXXXXXXX
Xxxxxxxx xx xxxxxxxxxxx:
1.&xxxx;Xxxxxx&xxxx;xxxx xxxxx xxxxxxxx xxxxxxxxxxx xxxxxxxx autologní, xxxxxxxx (xxxxxxxxx) xxxx xxxxxxxxxxx xxxx, xxxx xxxxxxxxx xxxxxxx a xxx xxxxxxxxx produktů jakéhokoliv xxxxxx do oběhového xxxxxxx.
2.&xxxx;Xxxxx xxxxxxxxx spotřeby, xxxxxxx xxxx dodávky xxxxxxx. Xxxxxx, xxx xx x xxxxxxxx xxxxx na xx:
xxxxxxxxxxxxxxxxxxx; xxxxxxxxxxx (RSR13), voxelotora xxxxxxxxxxxx xxxxxxxxxxxxx xxxxxxxx, xxxx. xxxxxx náhražky xxxxxxxx na hemoglobinu x xxxxxxxxxxxxxxxxxx hemoglobiny, xxxxxxx inhalační suplementace xxxxxxxx xxxxxxxx xxxx.
3.&xxxx;Xxxxxxxxx xxxxx intravaskulární xxxxxxxxxx x xxxx xxxx x krevními xxxxxxxxxxxx xxxxxxxxxxx nebo xxxxxxxxxx xxxxxxx.
X2.&xxxx;XXXXXXXX X XXXXXXXXX XXXXXXXXXX
Xxxxxxxx xx následující:
1. Podvádění, xxxx&xxxx;Xxxxx&xxxx;x&xxxx;Xxxxxx, xx účelem xxxxxxx integritu x xxxxxxxx&xxxx;Xxxxxx&xxxx;xxxxxxxxxx xxx&xxxx;Xxxxxxxxx kontrole.
Včetně, xxx xx x xxxxxxxx xxxxx xx xx:
Xxxxxx a/nebo úprava Vzorku, xxxx. přidáním proteáz xx&xxxx;Xxxxxx.
2.&xxxx;Xxxxxxxxxx xxxxxx x/xxxx xxxxxxx xxxx xxx xxxxxx 100 xx xx 12 hodin xxxxx xxxxxx xxxxxxxxx xxxxxxxxx x xxxxxxx xxxxxxxxxxxx ošetření, xxxxxxxxxxxxx xxxxxxx nebo xxxxxxxxxx xxxxxxxxxxxxxx xxxxxxxxx.
X3.&xxxx;XXXXXX X XXXXXXX DOPING
Z důvodu xxxxxxxxxxxxxxx xxxxxxx xxxxxxxxxxx xxxxxx je xxxxxxxx xxxxxxxxxxx:
1.&xxxx;Xxxxxxx xxxxxxxxxx kyselin xxxx xxxxxx xxxxxxx, xxxxx mohou xxxxxx xxxxxxxx genomu a/nebo xxxxxxx xxxx xxxxxxxxxx xxxxxxxxxxx. To xxxxxxxx, xxx xxxx xxxxxxx xxxxx xx technologie xxxxx xxxx, xxxxxxxxx xxxx x xxxxxxxxxxx xxxxxxx xxxx.
2.&xxxx;Xxxxxxx xxxxxxxxxx xxxx xxxxxxxxx xxxxxxxxxxxxxx xxxxx.
X6 XXXXXXXXXXX
XXXXXXXX XXX XXXXXXX
Xxxxxxx xxxxxxxx látky x této xxxxxxxxx xxxx xxxxxxxxxx xx&xxxx;Xxxxxxxxxx xxxxx, xxxxx látek xxxxxxxxx v S6.A, xxx jsou Nespecifické xxxxx.
Xxxxxxxx xxxxx&xxxx;x xxxx xxxxxxxxx xxxx: xxxxxx x xxxxxxxxxxxxxxxxxxxxxxxx (XXXX/"xxxxxx").
Xxxxxxx xxxxxxxxxxx xxxxxx všech xxxxxx xxxxxxxxxx xxxxxxxxx isomerů, xxxx.&xxxx;x-&xxxx;x&xxxx;x-, jsou zakázaná. Xxxxxxxxxxx zahrnují:
A: XXXXXXXXXXXX XXXXXXXXXXX
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxx xxxxxxx [4-fenylpiracetam (xxxxxxxxx)]
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxxxx(x-)
•&xxxx;x-xxxxxxxxxxxxxx
•&xxxx;xxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxx
Xxxxxxxxxxxx, xxxxx není xxxxxxxx v této xxxxxxxxx, xx&xxxx;Xxxxxxxxxxx látkou.
B: XXXXXXXXXX XXXXXXXXXXX
Xxxxxx, ale xx x xxxxxxxx xxxxx na xx:
•&xxxx;3-xxxxxxxxxx-2-xxxx (1,2-xxxxxxxxxxxxxxxxx)
•&xxxx;4-xxxxxxxxxxxxxxxxxx
•&xxxx;4-Xxxxxxxxxxx-2-xxxxx (xxxxxxxxxxxxxxxxx, 1.3-xxxxxxxxxxxxxxxxx, 1,3 XXXX)
•&xxxx;4-xxxxxxxxxxx-2-xxxx (1,3-xxxxxxxxxxxxxxxx)
•&xxxx;5-Xxxxxxxxxxx-2-xxxxx (1,4-xxxxxxxxxxxxxxxxxxx, 1.4-xxxxxxxxxxxxxxxxx, 1.4-XXXX)
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxxxxxx (xxxxxxxxxxxxxxxx)
•&xxxx;xxxxxxx***
•&xxxx;xxxxxxxxx (adrenalin)****
• etamivan
• etylamfetamin
• etylefrin
• etylefrin
• etylfenidát
• famprofazon
• fenbutrazát
• fenetylamin x xxxx xxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxx (xxxxxxxxx)
•&xxxx;xxxxxxxxxxxxxxxx (xxxxxxxxxxxxxxxxxxxx)
•&xxxx;xxxxxxxxxxxx
•&xxxx;xxxxx**
•&xxxx;xxxxxxx x xxxx xxxxxxx, např. xxxxxxxx, xxxxxxxx x α-xxxxxxxxxxxxxxxxxxxxx
•&xxxx;xxxxxxxxxxxxxxx
•&xxxx;xxxxxxxxxxxx
•&xxxx;xxxxxxxxxxxx***
•&xxxx;xxxxxxxxxxxxxxxxxxxxxxxx
•&xxxx;xxxxxxxxxxxxx [((±)-xxxxx-2-(xxxxxxxx-2-xx)-2-(xxxxxxxxx-2-xx)xxxxxx]
•&xxxx;xxxxxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxx (1,5-dimetylhexylamin)
• oktopamin
• oxilofrin (xxxxxxxxxxxxx)
•&xxxx;xxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxxxxxx
•&xxxx;xxxxxxxxxxxxx*****
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxxxx (xxxxxxxxxxxxxxxxxxxxx)
•&xxxx;xxxxxxxxxxxxx
x xxxxx xxxxx x podobnou xxxxxxxxx xxxxxxxxxx xxxx podobnými xxxxxxxxxxxx účinky.
VÝJIMKY
• klonidin;
• deriváty xxxxxxxxxxx x xxxxxxx jejich xxxxxxx, xxxxxxx, xxxxxx xxxx xxxxxx xxxxxxx (xxxx. brimonidin, xxxxxxxxxx, xxxxxxxxxxx, indanazolin, xxxxxxxxx, xxxxxxxxxxxx, xxxxxxxxxx, xxxxxxxxxxxxx) x stimulancií zahrnutých xx Xxxxxxxxxxxxxx xxxxxxxx xxx xxx 2023*.
* xxxxxxxxx, xxxxxxxxxx, xxxxxxxxxxxxxxxxx, xxxxxx, xxxxxxx, xxxxxxxxx x xxxxxxxx: xxxx xxxxx xxxx zahrnuté xx Xxxxxxxxxxxxxx xxxxxxxx 2023 x xxxxxx xxxxxxxxxx za Zakázané xxxxx.
** xxxxx (x-xxxxxxxxxxxxxxxx) x xxxx x-xxxxxxx: je xxxxxxxx xxxxx xxx xxxxxxxxxxx xxxxx xxx 5 xxxxxxxxxx x 1 xx moči.
*** efedrin xxx xxxxxxxxxxxx: xxxx xxxxxxxx při xxxxxxxxxxx xxxxx xxx 10 xxxxxxxxxx x 1 xx xxxx.
****&xxxx;xxxxxxxxx (adrenalin): xxxx xxxxxxx xxx xxxxxxxx xxxxxx, xxxx. xxxxx, oční xxxxxxxx xxxx xxxx podání xxxxxxxx x lokálními xxxxxxxxxx.
*****&xxxx;xxxxxxxxxxxxx: xx xxxxxxx, xxxxx je xxxx xxxxxxxxxxx vyšší xxx 150 xxxxxxxxxx na xx xxxx.
X7 XXXXXXXXX
XXXXXXXX XXX SOUTĚŽI
Všechny xxxxxxxx xxxxx v xxxx xxxxxxxxx xxxx považovány xx&xxxx;Xxxxxxxxxx xxxxx.
Xxxxxxxx xxxxx&xxxx;x xxxx xxxxxxxxx xxxx: xxxxxxxxx (xxxxxx).
Xxxxxxxxxxx narkotika, xxxxxx xxxxxx xxxxxxxxxx xxxxx xxxxxxxxx xxxxxxx, xxxx.&xxxx;x-&xxxx;x&xxxx;x-&xxxx;xxxx xxxxxxxx.
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxxxxx
•&xxxx;xxxxxxxxx (xxxxxx)
•&xxxx;xxxxxxxx x jeho xxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxx
•&xxxx;xxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxx
X8 XXXXXXXXXXX
XXXXXXXX XXX XXXXXXX
Xxxxxxx xxxxxxxx xxxxx x xxxx xxxxxxxxx xxxx xxxxxxxxxx za Specifické xxxxx.&xxxx;Xxxxxxxx xxxxx&xxxx;x této xxxxxxxxx xxxx: xxxxxxxxxxxxxxxxxxx (THC).
Všechny xxxxxxxx x xxxxxxxxxx xxxxxxxxxxx xxxx xxxxxxxx, xxxx.
•&xxxx;x konopí (hašiš, xxxxxxxxx) a konopných xxxxxxxxxx;
•&xxxx;xxxxxxxx x syntetické xxxxxxxxxxxxxxxxxxxxx (XXX);
•&xxxx;xxxxxxxxxx xxxxxxxxxxx xxxxxxxxxxxx xxxxxx XXX.
XXXXXXX
•&xxxx;xxxxxxxxxx
X9 XXXXXXXXXXXXXXX
XXXXXXXX PŘI XXXXXXX
Xxxxxxx xxxxxxxx xxxxx v xxxx kategorii xxxx xxxxxxxxxx xx&xxxx;Xxxxxxxxxx xxxxx.
Xxxxxxx xxxxxxxxxxxxxxx xxxxxxxx injekčně, xxxxxxxx xxxx perorálně (xxxxxx xxxx. xxxxxxx, xxxxxxxxxx, sublingvální) jsou xxxxxxxx.
Xxxxxx, xxx ne x xxxxxxxx pouze xx xx:
•&xxxx;xxxxxxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxxxxx
•&xxxx;xxxxxxxx
•&xxxx;xxxxxxxxxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxxxx acetonid
! XXXXXXXX
•&xxxx;Xxxx způsoby podání (xxxxxx xxxxxxxxxxx a xxxxxxxxx: xxxxxxxx-xxxxxxxxxxxx, xxxxxxxx, xxxxxxxxxxxx, oftalmologické, xxxx x xxxxxxxxxx) xxxxxx xxxxxxxx, pokud xxxx xxxxxxxxx v xxxxx xxxxxxxxxxxx dávek x xxxxxxxxxxxxxx xxxxxxxx xxxxxxxx.
X1 XXXX-XXXXXXXXX
XXXXXXXX X URČITÝCH XXXXXXXX
Xxxxxxx xxxxxxxx xxxxx x xxxx kategorii xxxx xxxxxxxxxx za Specifické xxxxx.
Xxxx-xxxxxxxxx xxxx zakázány xxxxx x xxxxxxxxxxxxx xxxxxxxx&xxxx;Xxx Xxxxxxx&xxxx;x kde xx xx označeno xxxxxxxx (*) i Mimo xxxxxx.
•&xxxx;xxxxxxxxxxxx sport (FIA)
• biliard (xxxxxxx disciplíny) (XXXX)
•&xxxx;xxxx (XXX)
•&xxxx;xxxxxxxxxxx (WA)*
• mini-golf (XXX)
•&xxxx;xxxxxxx (XXXX, XXX)*
•&xxxx;xxxxx (XXX)
•&xxxx;xxxxxxxx/xxxxxxxxxxxx (XXX) - xxxxx xx xxxxxx, xxxxxxxxxxx xxxxxxxx xxxxx/X-xxxxx x xxxxxxxxx X-xxxxx/"xxx xxx"
•&xxxx;xxxxxxxx xxxxxx (XXXX) x xxxxxxxxxxxx xxxxxxx potápění, xxxx ryb na xxxxxx a xxxxxxx xx xxxx
*&xxxx;xxxxxxxx xxxx&xxxx;Xxxx xxxxxx.
Xxxxxx, xxx ne x xxxxxxxx pouze xx xx:
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxx
•&xxxx;xxxxxxxxx
•&xxxx;xxxxxxxxxx
•&xxxx;xxxxxxxx
•&xxxx;xxxxxxxxxxx
•&xxxx;xxxxxxx
•&xxxx;xxxxxxx
XXXXXXXX
(±)-Xxxxxx-2-(xxxxxxxxxx-2-xx)-2-(xxxxxxxxx-2-xx)xxxxxxx, 15
1-Xxxxxxxxxxxxxx, 5
1-Xxxxxxxxxxxxxxx, 5
1-Xxxxxxxxxxxx, 5
1-Xxxxxxxxxxxxxxx, 5
1-Xxxxxxxxxxxx, 5
1.2-Xxxxxxxxxxxxxxxxxxx, 15
[1,2]Xxxxxxx[4’,5’:2,3]xxxxxx-4-xx-20-xx-17α-xx), 5
1.3-Xxxxxxxxxxxxxxxxx (1,3 DMAA), 15
1.3-Xxxxxxxxxxxxxxxxxx, 15
1.4-Xxxxxxxxxxxxxxxxx (1,4-XXXX), 15
1.4-Xxxxxxxxxxxxxxxxxxx, 15
1.5-Xxxxxxxx-xxxxxxxxxx, 15
2-Xxxxxxxxxxx, 10
2-Xxxxxxxxxxxx, 10
3α-Hydroxy-5α-androst-1-ene-17-one, 5
3ß-Hydroxy-5α-androst-1-ene-17-one, 5
3ß-Xxxxxxx-5α-xxxxxxxxx-17-xxx, 5
3ß-Hydroxyandrost-5-en-17-one, 6
3-Xxxxxxxxxxx, 10
3-Xxxxxxxxxxxx, 10
3-Xxxxxxxxxxx-2-xxxxx, 15
4-Xxxxxxxxxx-3,6,17 xxxxxx, 10
4-Xxxxxxxxxxxxxx, 5
4-Xxxxxx-17ß-xxxxxxx-17α-xxxxxxxxxxxxxx-1,4-xxxx-3-xxx, 5
4-Xxxxxx-17ß-xx-xxxx-4-xx-3-xxx, 6
4-Fluoromethylphenidate, 15
4-Xxxxxxxxxxxxxxxxxxx, 5
4-Xxxxxxxxxxx-2-xxxxx, 15
4-Xxxxxxxxxxxx-2-xxxxx, 15
4-Xxxxxxxxxxxxxxx, 14
4,17ß-Xxxxxxxxxxxxxxxx-4-xx-3-xxx, 5
5α-Androst-1-ene-3, 17-xxxxx, 5
5α-Xxxxxxx-1-xxx-3ß, 17ß-xxxx, 5
5α-Xxxxxxx-2-xx-17-xx, 10
5α-Xxxxxxx-2-xx-17-xxx, 10
5α-Androst-3-en-17-ol, 10
5α-Xxxxxxx-3-xx-17-xxx, 10
5α-Xxxxxxxxxxxxxxxxxxx, 5
5-Androstenedione, 5
5-Xxxxxxxxxxx-2-xxxxx, 15
6-Xxx, 10
7α-Hydroxy-DHEA, 5
7ß-Xxxxxxx-XXXX, 5
7-Xxxx-XXXX, 5
11-Xxxxxxxxxxxxxxxxxxx, 5
17α-Xxxxxx[1,2,5]xxxxxxxxxx[3’,4’:2,3]-5α-xxxxxxxxx-17ß-xx, 5
17α-Xxxxxx-5α-xxxxxxx-2-xx-17ß-xx, 5
17α-Xxxxxx-5α-xxxxxxx-3-xx-17ß-xx, 5
17α-Xxxxxxxxxxxxxxxxxxx, 5
17ß-Xxxxxxx-2α,17α-xxxxxxxx-5α-xxxxxxxxx-3-xxx, 6
17ß-Xxxxxxx-5α-xxxxxxx-1-xx-3-xxx, 5
17ß-Xxxxxxx-5α-xxxxxxxxx-3-xxx, 5
17ß-Xxxxxxx-5ß-xxxxxxxxx-3-xxx, 5
17ß-xxxxxxx-17α-xxxxxx-5α-xxxxxxx-1-xx-3-xxx, 6
17ß-Xxxxxxx-17α-xxxxxxxxxxxxxx-1,4-xxxx-3-xxx, 6
17ß-Xxxxxxx-17α-xxxxxxxxxx-4-xx-3-xxx,6
17ß-Xxxxxxx-17α-xxxxxxxxxxx-4,9-xxxx-3-xxx, 6
17ß-Xxxxxxx-17α-xxxxxxxxxxx-4,9,11- xxxxx-3-xxx, 6
17ß-Xxxxxxxxxxx-4,9,11-xxxxx-3-xxx, 6
17ß-[(Tetrahydropyran-2-yl)oxy]-1’H-pyrazolo[3,4:2,3]-5α-androstane, 6
17-Xxxxxxx-18x-xxxx-19-xxx-17α-xxxxxx-4,9,11-xxxxx-3-xxx, 6
19-Xxxxxxxxxxxxxxxxx, 5
19-Xxxxxxxxxxxxxxxxxx, 5
19-Norpregna-4-en-17α-ol, 5
19-Xxxxxxxxxxxxxxx, 6
a-Pyrrolidinovalerophenone, 15
X
XXX-031, 11
Xxxxxxxxxx, 19
Xxxxxxxxxxxxx, 12
Xxxxxxx X-xxxxxxxxxxxx xxxxxxxxxx, 11
Xxxxxxx xxxxxxxx XXX xxxxxxxxxxx, 11
Xxxxxxxxx, 14
Adrenaline, 15
Adrenosterone, 5
XXXXX, 11
Albumin, 12
Alexamorelin, 8
Xxxxxxxxxx, 19
Xxxxxxxxxxx, 14
Xxxxxxxxxx, 14
Xxxxxxxxxxx, 14
Xxxxxxxxx, 12
Xxxxxxxxxxxxxxxxx, 10
Xxxxxxxxxxxx, 14
AMP-activated xxxxxxx xxxxxx (XXXX), 11
Xxxxxxxxxx, 8
Xxxxxxxxxxx, 10
Xxxxxxxx, 6
Xxxxxxx-4-xxx-3ß,17ß-xxxx, 5
Xxxxxxx-4-xxx-3,11,17- xxxxxx, 5
Androst-4-ene-3,17-dione, 5
Xxxxxxx-5-xxx-3ß,17ß-xxxx, 5
Xxxxxxx-5-xxx-3,17-xxxxx, 5
Androsta-1,4,6-triene-3,17-dione, 10
Xxxxxxxx-1,4-xxxxx-3,17-xxxxx, 5
Xxxxxxxx-3,5-xxxxx-7,17-xxxxx, 10
Xxxxxxxxxxxxxx, 5
Xxxxxxxxxxxxxxxxxxx, 10
Androstenediol, 5
Xxxxxxxxxxxxxxx, 5
Xxxx-xxxxxxx xxxxxxxx XXX xxxxxxxxxx, 11
XXX-9604, 7
Xxxxxxxxxxx, 11
Xxxxxxxxxxxx, 9
Xxxxxxxxxx, 10
Xxxxxx XXX, 7
Xxxxxxxx, 19
X
Xxxxxxxxxxxx, 10
Xxxxxxxxxxxxx, 18
Xxxxxxxxxxxxxxxxxxx, 12
Benfluorex, 14
Xxxxxxxxxxxx, 15
Benzylpiperazine, 14
Xxxxxxxxxxxxx, 18
Xxxxxxxxx, 19
Bimagrumab, 11
Xxxxxxxxxx, 19
Xxxxx, 13
Blood (xxxxxxxxxx), 13
Xxxxx (xxxxxxxxxx), 13
Xxxxx (xxxxxxxxxxxx), 13
Blood (homologous), 13
Xxxxx xxxxxxxxxxxx, 13
Xxxxxxxxxxx, 5
Xxxxxxxxx, 5
Boldione, 5
XXX-157, 4
Xxxxxxxxxxx, 15
Xxxxxxxxxxxx, 12
Xxxxxxxxx, 14
Xxxxxxxxxx, 18
Bumetanide, 12
Xxxxxxx, 19
Xxxxxxxxxxxxx, 16
Buserelin, 8
X
Xxxxxxxxxxx, 5
Xxxxxxxxxxx, 17
Xxxxxxxx, 17
Xxxxxxxxx, 12
Xxxxxxxxxxxx EPO (XXXX), 7
Xxxxxxxxx, 14
Xxxxxxxxx, 19
Xxxxxxxxxx, 19
Xxxxxxx, 12,15
Cathinone, 15
Xxxxxxxxxx, 19
Xxxx (doping), 13
Xxxx (xxxxxxxxxxx xxxxxxxx), 13
Cell (xxxxxx), 13
Xxxx (xxx xxxxx), 13
Chlorothiazide, 12
Chlortalidone, 12
Xxxxxxxxx Gonadotrophin (CG), 8
Xxxxxxxxxxx, 18
XXX-1293, 8
CJC-1295, 8
Xxxxxxxxxxx, 6
Xxxxxxxxxxx, 14
Clomifene, 10
Xxxxxxxxxxx, 15
Clonidine, 15
Clostebol, 5
XXXX-530, 7
Xxxxxx, 7
Xxxxxxx, 14
Xxxxxxxxxxxx, 8
Xxxxxxxxxxxxxxx, 8
Xxxxxxxxx, 18
Xxxxxxxxxxxx, 14
Xxxxxxxxxxx, 14
Xxxxxxxxxx, 10
X
Xxxxxxx, 5
Daprodustat, 7
Xxxxxxxxxxxx (xXXX), 7
Deflazacort, 18
Xxxxxxxxxxxxxxxxxxxxxxxxxxxxxx, 5
Xxxxxxxxxxxxxxxxxxxxxx (XXXX), 6
Xxxxxxxxxx, 8
Xxxxxxxxxxxx, 12
Xxxxxxxxxxxxxxxxxxxxxxxx, 5
Xxxxxxxxxxxxx, 18
Xxxxxxx, 12
Xxxxxxxxxxxxxx, 16
Xxxxxxxxxxx, 16
Xxxxxxxxxxxxxxx, 15
Xxxxxxxxxxxxxxxxxxx, 15
Xxxxxxxxxxxx, 11
Xxxxxxxxxxx, 12
Xxxxxxxxxxxx, 12
Drostanolone, 5
X
Xxxxxxx, 14
Xxxxxxxxxxx (XXX13), 13
Xxxxxxxxx, 6
Xxxxxxxxx, 12,15
Xxxxxxxxxxxxxxx, 5
Xxx-xxxxxxxxxxxxxxxxxxx, 5
Xxxxxxxxxxx, 15
Xxxxxxxx, 5
Xxxxxxxxxxxxxxx, 5
XXX-xxxxx xxxxxxxxxx, 7
XXX-Xx, 7
XXX-xxxxxxx agents, 7
Xxxxxxxxxxxxxx xxxxxxxx xxxxxxxx, 7
Xxxxxxxxxxxxxxx (EPO), 7
Xxxxxxx, 19
Xxxx-4-xxx-3,17-xxxx, 5
Estr-4-ene-3,17-dione, 5
Etacrynic xxxx, 12
Etamivan, 15
Ethylestrenol, 5
Xxxxxxxxxxxxxx, 15
Xxxxxxxxxxxxxx, 15
Xxxxxxxxxx, 15
Xxxxxxxxxx, 8
Exemestane, 10
X
Xxxxxxxxxxxx, 15
Xxxxxxxxxxx, 12
Xxxxxxxxxxxx, 15
Fencamfamin, 15
Xxxxxxxxx, 14
Xxxxxxxxxxx, 14
Xxxxxxxxxxxx, 14
Xxxxxxxxx, 9
Fenoxazoline, 15
Xxxxxxxxxxx, 14
Xxxxxxxx, 16
Xxxxxxxxxx xxxxxx xxxxxxx (XXXx), 8
Xxxxxxxxxxx, 18
Xxxxxxxxxxxxx, 18
Xxxxxxxxx, 15
Fluoxymesterone, 5
Xxxxxxxxxxx, 18
Xxxxxxxxxxx, 11
Fonturacetam, 14
Xxxxxxxxxxx,5
Xxxxxxxxxx, 10
Xxxxxxxxxx, 9, 12
Xxxxxxxxxxx, 10
Xxxxxxxxx, 5
Furfenorex, 14
Xxxxxxxxxx, 12
G
GATA xxxxxxxxxx, 7
Xxxx xxxxxx, 13
Xxxx xxxxxxx, 13
Gene xxxxxxxxx, 13
Xxxx transfer, 13
Xxxxxxxxxx, 5
Xxxxxxx, 8GH-releasing xxxxxxxx (XXXXx), 8
Gonadorelin, 8
Goserelin, 8
Xxxxxx hormone (XX), 8
Xxxxxx xxxxxxx xxxxxxxxxxxxx (XXX), 8
XX1516,1
XX501516, 11
H
Haemoglobin (xxxxxxxx), 13
Haemoglobin (xxxxx xxxxx xxxxxxxxxxx), 13
Xxxxxxxxxxx (xxxxxxxxxxxxxxxxx products), 13
Xxxxxxx, 17
Xxxxxxxxxx xxxxxx xxxxxx (XXX), 8
Heptaminol, 15
Xxxxxx, 16
Xxxxxxxxx, 8
hGH 176-191, 8
Xxxxxxxxxx, 9
Xxxxxxxxxx, 15
Hydrochlorothiazide, 12
Xxxxxxxxxxxxxx, 18
Xxxxxxxxxxxxx, 16
Xxxxxxxxxxxxxxxxx, 15
Xxxxxxxxxxxx xxxxxx, 12
Xxxxxxx-xxxxxxxxx xxxxxx (HIF) xxxxxxxxxx xxxxxx, 7
X
Xxxxxxxxxxx, 15
Indacaterol, 9
Xxxxxxxxxxxx, 15
Xxxxxxxxxx, 12
Xxxxxxxxx, 13
Xxxxxxxxxx (&xx;100 mL), 13
Xxxxxx xxxxxx receptor xxxxxxxx, 7
Xxxxxxx-xxxx growth xxxxxx-1 (XXX-1), 8
Xxxxxxx-xxxxxxxx, 11
Xxxxxxxx, 11
Xxxxxxxxxxx xxxxxxxxx/xxxxxxxxxx, 13
XXX2, 7 Xxxxxxxxxx, 8
Xxxxxxxxxxxxx, 15
K
K-11706, 7
L
Labetalol, 19
Xxxxxxxxxxxxx, 11
Lenomorelin, 8
Xxxxxxxxx, 10
Xxxxxxxxxxx, 8
Xxxxxxxxxxxxxxxx, 15
Levosalbutamol, 9
XXX-4033, 6
Xxxxxxxxx, 6
Xxxxxxxxxxxxxxxx, 14
Xxxxxxxxxxxxxxxxx, 8
Xxxxxxxxxxxx, 7
Xxxxxxxxxxx xxxxxxx (LH), 8
X
Xxxxxxxxxxx, 8
Xxxxxxxx, 12
Xxxxxxxxx, 17
Mechano xxxxxx factors (XXXx), 8
Xxxxxxxxxxxxx, 15 Xxxxxxxxx, 14
Xxxxxxxxx, 11
Mephedrone, 15
Mephentermine, 14
Xxxxxxxx, 14
Mestanolone, 6
Mesterolone, 6
Xxxxxxxxxxxxx(x-), 14
Xxxxxxxxxxxx, 6
Metenolone, 6
Xxxxxxxxx, 16
Methandriol, 6
Xxxxxxxxxxxx, 6
Xxxxxxxxxx, 15
Xxxxxxx xxxxxxxxxxxx xxxxxx-xxxxxxx beta (XXXX), 7
Xxxxxx-1-xxxxxxxxxxxx, 6
Xxxxxxxxxxxxxxx, 6
Methyldienolone, 6
Xxxxxxxxxxxxxxxxxxxxxxxxx, 15
Xxxxxxxxxxxxxxxxxxxxxxxxxxxxx, 15
Xxxxxxxxxxxxxxx, 12, 15
Xxxxxxxxxxxxxxxxx, 15
Xxxxxxxxxxxxxxxx, 15
Xxxxxxxxxxxxxxxxxxxxx, 6
Methylphenidate, 15
Methylprednisolone, 18
Xxxxxxxxxxxxxxxx, 15
Xxxxxxxxxxxxxxxxxx, 6
Xxxxxxxxxxxxxxxx, 6
Xxxxxxxxxxxx, 19
Xxxxxxxxxx, 12
Xxxxxxxxxx, 19
Xxxxxxxxxxx, 6
Xxxxxxxxxx, 6
Xxxxxxxxx, 14
Xxxxxxxxxx, 7
Mometasone, 18
Xxxxxxxx, 16
Xxxxxxxxx inhibitors, 11
Myostatin xxxxxxxxx-xxxxxxxxxxxx xxxxxxxxxx, 11
Myostatin xxxxxxxxxx, 11
Xxxxxxxxx-xxxxxxx xxxxxxxx, 11
Xxxxxxxxx-xxxxxxxxxxxx antibodies, 11
X
Xxxxxxx, 19
Xxxxxxxxx, 8
Xxxxxxxxxx, 6
Naphazoline, 15
Xxxxxxxxx, 19
Xxxxxxxxxxxx, 16
Xxxxxxxxxxx, 15
Xxxxxxxxxxx, 6
Xxxxxxxxxxxx, 6
Xxxxxxxxxxxxxxx, 6
Xxxxxxxxxxxx, 15
Norfenfluramine, 14
Nucleic xxxxx, 13
Xxxxxxx xxxx xxxxxxxxx, 13
O
Octodrine, 15
Octopamine, 15
Xxxxxxxxxx, 9
Xxxxxxxxxxxx, 6
Ospemifene, 10
Xxxxxxxx, 6
Xxxxxxxxx, 6
Xxxxxxxxxxx, 6
Xxxxxxxxxx, 15
Xxxxxxxxxx, 19
Xxxxxxxxx, 16
Xxxxxxxxxxxx, 6
Oxymetazoline, 15
Xxxxxxxxxxxx, 6
Xxxxxxxxxxx, 16
P
Pamabrom, 12
Xxxxxxxxxxxxxxxxxxxxxx, 15
Xxxxxxxxxxxx, 7
Pemoline, 15
Pentazocine, 16
Xxxxxxxxxxx, 15
Perfluorochemicals, 13
Peroxisome xxxxxxxxxxxx xxxxxxxxx receptor xxxxx agonists, 11
Xxxxxxxxx, 16
Xxxxxxxxxxxxxxx, 14
Xxxxxxxxxxxxxx, 15
Xxxxxxxxxxxxx, 15
Xxxxxxxxxxxxxxxx, 15
Xxxxxxxxxxx, 14
Xxxxxxxx, 19
Xxxxxx xxxxxxxxx, 12
Xxxxxxxx-xxxxxxx xxxxxx factor (XXXX), 8
x-xxxxxxxxxxxxxxxx, 14
Xxxxxxxxxxx, 8
Xxxxxxxxxx, 6
Xxxxxxxxxxxx, 18
Prednisone, 18
Xxxxxxxxxxx, 14
Xxxxxxxxxx, 12
Procaterol, 9
Xxxxxxxxxx, 14
Xxxxxxxxxxx, 19
Xxxxxxxxxxxxxxx, 15
Xxxxxxxxxxx, 6
Xxxxxxxxx, 13
Xxxxxxxxxxxxxxx, 12, 15
X
Xxxxxxxxxx, 6
X
XXX140, 6
Xxxxxxxxxxx, 6
Xxxxxxxxxx, 10 , 9
Xxxxxxxxxx, 7
X
X-23, 6
Xxxxxxxxxx, 9, 12
Xxxxxxxxxx, 9
Xxxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxxxx (XXXXx), 6
Xxxxxxxxxx, 15
Sermorelin, 8
Xxxxxxxxxxx, 15
Xxxxxxxxxxxx, 15
Xxxxxxxxxxx, 8
Xxxxxxxxxx, 8
Sotalol, 19
Xxxxxxxxxxx, 7
Xxxxxxxxxxxxxx, 12
XX9009, 11
Xxxxxxxxxx, 11
Xxxxxxxxxx, 6
Stenbolone, 6
Xxxxxxxxxx, 15
X
Xxxxxxxxxxx, 8
Xxxxxxxxx, 10
Xxxxxxxxx, 13
XX-500, 8
Tenamfetamine, 15
Xxxxxxxxxxx, 9
Xxxxxxxxxxx, 8
Xxxxxxxxxxxx, 10
Testosterone, 6
Xxxxxxxxxxxxxxxxxxxxx, 17
Xxxxxxxxxxxxxxxxxxxx, 6
Xxxxxxxxxxx, 15
Xxxxxxxxx, 12
Xxxxxxxx-154, 8
Tibolone, 6
Xxxxxxx, 19
Tolvaptan, 12
Xxxxxxxxxx, 12
Xxxxxxxxxx, 10
Transforming xxxxxx xxxxxx xxxx (XXX-ß) xxxxxxxxxx xxxxxxxxxx, 7
Xxxxxxxxxx, 6
Xxxxxxxxxxx, 9
Xxxxxxxxxxxxx xxxxxxxxx, 18
Xxxxxxxxxxx, 12
Trimetazidine, 11
Xxxxxxxxxxxxx, 9
Xxxxxxxxxxx, 8
Xxxxxxxxxxxxxx, 15
Xxxxxxxxxxx, 9
X
Xxxxxxxxxx (XXX-6548), 7
Xxxxxxx, 12
Xxxxxxxx endothelial growth xxxxxx (VEGF), 8
Vilanterol, 9
Xxxxxxxxx, 13
X
Xxxxx, 7
Xylometazoline, 15
X
XX-11, 6
X
Xxxxxxx, 6
Xxxxxxxxxx, 6

XXXXXXX XXXXXXXXXXXXX KODEX
MEZINÁRODNÍ
STANDARD
PRO XXXXXXXXXXXX XXXXXXX
2023

Xxxxxxxxxxx xxxxxxxx xxx Xxxxxxxxxxxx výjimky
Mezinárodní xxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx výjimky v rámci Xxxxxxxxx xxxxxxxxxxxxxxx&xxxx;Xxxxxx&xxxx;xx xxxxxxxx&xxxx;Xxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxxxxxxxx jako součást Xxxxxxxxx antidopingového xxxxxxxx. Xxx xxxxxxxx xx xxxxxxxxxx xx&xxxx;Xxxxxxxxx, veřejnými xxxxxx a xxxxxxx xxxxxxxxxxx xxxxxxxxxxxx stranami.
Mezinárodní xxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx xxxxxxx&xxxx;xxx xxxxxx xxxxxx x roce 2004 a xxxxx xxxxxxxxx 1. ledna 2005. Následně byl xxxxxxxx xxxxxxxxxxx, xxxxxx x xxxxxxxxx od xxxxx 2009, xxxxxxx x xxxxxxxxx od xxxxx 2010, xxxxxxx x xxxxxxxxx xx xxxxx 2011, počtvrté x xxxxxxxxx od xxxxx 2015, xxxxxx x účinností xx xxxxx 2018, xxxxxxx x xxxxxxxxx xx xxxxx 2019, xxxxxxx x xxxxxxxxx xx xxxxx 2021. Tato xxxxxxxxxx xxxxx byla xxxxxxxxx XX&xxxx;XXXX&xxxx;23. xxxx 2022 x je xxxxxx od 1. xxxxx 2023.
&xxxx;
Xxxxxx:
XXXX
Xxxxx Xxxxxxxx Xxxxx
800 Xxxxx Victoria (Xxxxx 1700)
XX Xxx 120
Xxxxxxxx, Xxxxxx
Xxxxxx X4X 1X7
Xxx: + 1 514 904 9232
Xxx: + 1 514 904 8650
E-mail: xxxx@XXXX-xxx.xxx
&xxxx;
XXXXX
|
XXXXX XXXX: ÚVOD, XXXXXXXXXX&xxxx;XXXXXX, XXXXXXXXXX&xxxx;XXXXXXXXXXXXX STANDARDU A XXXXXXXX |
|
|
1.0 |
Xxxx x xxxxxxxxx |
|
2.0 |
Xxxxxxxxxx&xxxx;Xxxxxx |
|
3.0 |
Xxxxxxxx a xxxxxx xxxxx |
|
XXXXX XXXX: XXXXXXXXX X POSTUP XXX UDĚLOVÁNÍ TV |
|
|
4.0 |
Udělování TV. |
|
5.0 |
Povinnosti Antidopingových organizací týkající xx&xxxx;XX |
|
6.0 |
Xxxxxx xxx xxxxxxxx xxxxxxx x&xxxx;XX |
|
7.0 |
Xxxxxx při xxxxxxxx&xxxx;XX |
|
8.0 |
Xxxxxxxxxxx xxxxxxxxxx x&xxxx;XX&xxxx;xx xxxxxx XXXX |
|
9.0 |
Xxxxxxxxx xxxxxxxxx |
|
XXXXXXX 1: Xxxxxxxxx xxxxxxx xxx xxxxxx 4.4&xxxx;Xxxxxx |
|
XXXX XXXXX: XXXX, XXXXXXXXXX XXXXXX, XXXXXXXXXXXXX STANDARDU X XXXXXXXX
1.0&xxxx;Xxxx x xxxxxxxxx
Xxxxxxxxxxx xxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx xxxxxxx&xxxx;xx xxxxxxxx&xxxx;Xxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxxxxxxxx xxxx xxxxxxx Světového xxxxxxxxxxxxxxx xxxxxxxx.
Xxxxxx&xxxx;Xxxxxxxxxxxxx standardu pro Terapeutické xxxxxxx&xxxx;xx xxxxxxxx (x) xxxxxxxx, xxxxx xxxx xxx xxxxxxx, xxx xxxx xxxxxxx&xxxx;Xxxxxxxxxxxx xxxxxxx&xxxx;(xxxx&xxxx;XX), jež xxxxxxxx xxxxxxxxxx&xxxx;Xxxxxxxx xxxxx&xxxx;xx&xxxx;Xxxxxx Xxxxxxxxx&xxxx;xxxx&xxxx;Xxxxxxx&xxxx;xx&xxxx;Xxxxx x xxxxxxx,&xxxx;Xxxxxx&xxxx;x/xxxx&xxxx;Xxxxxx&xxxx;xx&xxxx;Xxxxx x Xxxxxx Zakázané xxxxx&xxxx;xxxx&xxxx;Xxxxxxxx xxxxxx Xxxxxxxxxx&xxxx;xxx&xxxx;xxxxxxxxxxxx&xxxx;xxxxx; (x) xxxxxxxxxx uložené Antidopingovým xxxxxxxxxxx&xxxx;xxxxxxxx xx xxxxxxxxxxx x&xxxx;XX&xxxx;x xxxxxx oznamování; (x) xxxxxx, xxxxx xx&xxxx;Xxxxxxxxx&xxxx;xxxxx x&xxxx;XX; (x) xxxxxx, jakým xx&xxxx;Xxxxxxxxx&xxxx;xxxxxx&xxxx;XX&xxxx;xxxxxxxx xxxxxx&xxxx;Xxxxxxxxxxxxxx xxxxxxxxxx&xxxx;x uznávanou xxxxx&xxxx;Xxxxxxxxxxxxxx xxxxxxxxxx; (e) xxxxxx, xxxxx&xxxx;XXXX&xxxx;xxxxxxxxxxx xxxxxxx&xxxx;XX; x (x) ustanovení x přísné xxxxxxxxxx, xxxxx jsou xxxxxxxxxx x xxxxxxx xxxxxxxxx&xxxx;XX.
Xxxxxxx xxxxxxxxx x xxxxx&xxxx;Xxxxxxxxxxxx xxxxxxxxx, xxxxx jsou xxxxxxxxxx x Xxxxxx, xxxx psány xxxxxxxx. Xxxxxxx, které xxxx xxxxxxxxxx x xxxxx xx jiném Mezinárodním xxxxxxxxx, xxxx xxxxxxxxx.
2.0&xxxx;Xxxxxxxxxx&xxxx;Xxxxxx
Xxxxxxxxxxx xxxxxx&xxxx;Xxxxxx&xxxx;2021 xx xxxxx xxxxxx&xxxx;Xxxxxxxxxxxxx xxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx výjimky, xxx xx xxxxxx odkazem xx xxxxxxx&xxxx;Xxxxx:
•&xxxx;Xxxxxx 4.4&xxxx;Xxxxxx Xxxxxxxxxxxx xxxxxxx&xxxx;("XX")
•&xxxx;Xxxxxx 13.4&xxxx;Xxxxxx&xxxx;Xxxxxxxx xxxxxxxx se TV
3.0 Definice x xxxxxx xxxxx
3.1&xxxx;Xxxxx definované x Kodexu 2021, xxxxx xxxx použité x&xxxx;Xxxxxxxxxxxx xxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx výjimky
ADAMS (ADAMS): Xxxx-Xxxxxx Xxxxxxxxxxxxxx xxx Xxxxxxxxxx Xxxxxx): xx xxxxxxx&xxxx;XXXX&xxxx;xxx správu databáze xxxx webové rozhraní xxx xxxxxxxx xxx, xxxxxx xxxxxxxx, xxxxxxx x xxxxxxx, vytvořený xx xxxxx zúčastněným xxxxxxx x&xxxx;XXXX&xxxx;x xxxxxx xxxxxxxxxxxxxxx činnostech, ve xxxxxxx x legislativní xxxxxxxx xxxxx.
Xxxx&xxxx;(Xxxxx): Série xxxxxxxxxxxx&xxxx;Xxxxxxx&xxxx;xxxxxxxxxxxxx xxxxxxxx, xxxxx xx xxxxxx xxxxxx xxxxxxx (např. olympijské xxx, xxxxxxxxxxx xxxxx Xxxxxxxxxxx xxxxxxxx, Panamerické xxx).
Xxxxxxxxxxxxx organizace (Anti-Doping Organization): WADA nebo Signatář, xxxxx xxxxxxxx xx xxxxxxx xxxxxxxx pro xxxxxxxxxx, xxxxxxxxxxxx xxxx xxxxxxxxxxx jakékoli xxxxx xxxxxxx&xxxx;Xxxxxxxxx kontroly. Xxxxx xx např. x Xxxxxxxxxxx xxxxxxxxxx xxxxx, Xxxxxxxxxxx xxxxxxxxxxxxx výbor, xxxx&xxxx;Xxxxxxxxxxxx xxxxxxxxxx xxxx, xxxxx xxxxxxxxx&xxxx;Xxxxxxxxx&xxxx;x průběhu xxxxx&xxxx;Xxxx, Xxxxxxxxxxx xxxxxxxx x&xxxx;Xxxxxxx Antidopingové xxxxxxxxxx.
XXX:&xxxx;(Xxx Xxxxx xx Arbitration xxx Xxxxx): Xxxxxxxx xxxx xxx xxxxx.
Xxxxxx&xxxx;(Xxxxxxxxxx): Xxxxxxxx, xxxxxxx&xxxx;Xxxxxx, xxxxxxxx xxxxxxx&xxxx;Xxxxxx&xxxx;(xx xxxxxxx xxxx xxxxx xxx xxxxx, xxxxx má xxxxx xxxxxxx&xxxx;Xxxxx&xxxx;xxxxxxxx xxxxxxxx xxxx xxxx mít kontrolu xxx&xxxx;Xxxxxxxxx xxxxxx&xxxx;xxxx&xxxx;Xxxxxxxxx metodou nebo xxx xxxxxxxxx, ve xxxxxx xx Xxxxxxxx xxxxx nebo Xxxxxxxx xxxxxx xxxxxxxxx), avšak x xxx, xx xxxx&xxxx;Xxxxx&xxxx;xxxx výlučnou xxxxxxxx xxx Zakázanou xxxxxx xxxx Xxxxxxxxx xxxxxxx xxxx xxx xxxxxxxxx, xx xxxxxx xx&xxxx;Xxxxxxxx xxxxx&xxxx;xxxx&xxxx;Xxxxxxxx xxxxxx&xxxx;xxxxxxx, xxxx xxxxxxx&xxxx;Xxxxxx&xxxx;xxxxxxxxxx xxxxx xx&xxxx;Xxxxx, xxxxx xxxxxx x xxxxxxxxxxx&xxxx;Xxxxxxxx xxxxx&xxxx;xxxx&xxxx;Xxxxxxxx xxxxxx&xxxx;x xxxx v úmyslu xxxxxxx xxxxxxxx xxx xxxx xxxxxxxxx. X xxxxxxxx antidopingového xxxxxxxx, xxxxx xxx xxxxx x&xxxx;Xxxxxx, xxxxxxx, xxxxxxxx xxxx xxxxxxxxx xxxxxxxxxx xxxxxxxx, xx&xxxx;Xxxxx&xxxx;xxxxxxxx xxxxxxxxxxxxx xxxxxxxx, xxxxx tato Osoba konkrétní xxxxx, kterými prokáže, xx nikdy neměla Držení v xxxxxx x xxxxxxxx xx&xxxx;Xxxxxx&xxxx;xxx xxxxxxxxxx xxx&xxxx;Xxxxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxxxx. Xxx xxxxxx xx xxxxxx xxxxxxxx x této xxxxxxxx, xxxxx (xxxxxx elektronického xx jiného xxxxxxx)&xxxx;Xxxxxxxx xxxxx&xxxx;xxxx&xxxx;Xxxxxxxx xxxxxx&xxxx;xxxxxxxxxxx xxxx&xxxx;Xxxxxx&xxxx;xxx&xxxx;Xxxxx, xxxxx xxxxx xxxxxxxxx.
[Xxxxxxxx x Xxxxxx: Podle xxxx xxxxxxxx by xxxxxxxx xxxxxxxxxxxx xxxxxxxx x autě Xxxxxxxxx xxxxxxxxx xxxxxxxx, xxxxx Xxxxxxxxx xxxxxxxxx, xx xxxx xxxxxxxx někdo xxxx. V tom xxxxxxx xxxx Xxxxxxxxxxxxx xxxxxxxxxx xxxxxxx, xx x když Sportovec xxxxx xxxxxxxx xxxxxxxx xxx xxxxx, věděl x xxxxxxxxxxxx steroidech x xxx x xxxxxx je xxxxxx. Xxxxxxx x případě xxxxxxxxxxxx xxxxxxxx xxxxxxxxxx x domácí lékárničce, xx xxxxx má xxxxxxx Xxxxxxxxx x xxxx xxxxxxx/xxxxxxxxx, musí Xxxxxxxxxxxxx xxxxxxxxxx xxxxxxx, xx Xxxxxxxxx x xxxxxxxxxx x xxxxxxxxxx xxxxx x xx xxx v úmyslu xx použít. Samotný xxxxxx xxxxxx Zakázané xxxxx xxxxxxxxxxx sám x xxxx Xxxxxx, x když například xxxxxxx xxxxxxxx, xxxxxx xxx xxxxx xxxx xxxx xx xxxxxx xx adresu xxxxx xxxxx.]
Xxxxx&xxxx;(Xxxx): Světový antidopingový Kodex.
Mezinárodní Xxxx&xxxx;(Xxxxxxxxxxxxx Xxxxx):&xxxx;Xxxx&xxxx;xxxx&xxxx;Xxxxxx, xxxxxx xxxx Xxxxxxxxxxx olympijský xxxxx, Xxxxxxxxxxx xxxxxxxxxxxxx xxxxx, Xxxxxxxxxxx federace, Organizátor xxxxxxxx Akce nebo xxxx xxxxxxxxxxx sportovní xxxxxxxxxx, xxxx&xxxx;Xxxx, na xxxxxx xxxxx x uvedených xxxxxxxxxx jmenuje technické xxxxxxxxxxx.
Xxxxxxxxxxx xxxxxxxx&xxxx;(Xxxxxxxxxxxxx Xxxxxxxx): Xxxxxxxx, xxxxx xxxxxxx&xxxx;XXXX&xxxx;xx xxxxxxx&xxxx;Xxxxxx. Dodržování Mezinárodního standardu (na xxxxxx xx jiných xxxxxxxxxxxxxx xxxxxxxxx, xxxxxxx xxxx xxxxxxxx) xxxx xxxxxxxxxx x závěru, xx xxxxxxx, které xxxx xxxxxxx v Mezinárodním xxxxxxxxx, byly xxxxxxxxxxx xxxxx.&xxxx;Xxxxxxxxxxx xxxxxxxxx&xxxx;xxxxxxxx xxxxxxx&xxxx;Xxxxxxxxx xxxxxxxxx&xxxx;xxxxxx podle Mezinárodních xxxxxxxxx.
Xxxx Xxxxxx&xxxx;(Xxx-xx-Xxxxxxxxxxx): Jakékoli xxxxxx, xxxxx xxxx&xxxx;Xxx Xxxxxxx.
Xxxxxxxxx x výsledky (Results Xxxxxxxxxx): Xxxxxx xxxxxxxxxxx časový xxxxx xxxx xxxxxxxxxxx xxxxx článku 5&xxxx;Xxxxxxxxxxxxx xxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxx s xxxxxxxx&xxxx;xxxx x jistých xxxxxxxxx (xxxx.&xxxx;Xxxxxxxx nález, Biologický xxx Xxxxxxxxx, xxxxxxxxxxxx xxxxxxxxx o xxxxx xxxxxx) a xxxx xxxxx xxxx vyrozuměním xxxxxxxx xxxxxxxxxxx x xxxxxx 5 Mezinárodního xxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxx x xxxxxxxx, xxxx xxxxxxxx xx xx xxxxxxx vyřešení xxxxxxx, xxxxxx ukončení xxxxxx x xxxxx xxxxxxxx xxxx na xxxxxx xxxxxxxx (xxxxx bylo xxxxxxxx xxxxxx).
Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx&xxxx;(Xxxxxxxx Anti-Doping Xxxxxxxxxxxx): Xxxxxxx xx subjekty xxxxxx v xxxxx xxxx, které xxxx xx xxxxxxx úrovni xxxxxxxx xxxxxxxxx a xxxxxxxxxxx přijímat a xxxxxxxxx xxxxxxxxxxxxx pravidla, xxxxxxxxx xxxxxx&xxxx;Xxxxxx, Xxxxxxxx x výsledky testů x vést xxxxxx. Xxxxxxxx xxxx stanovení xxxxxx provedeno xxxxxxxxxx xxxxxxx xx xxxxxx xxxxxx správy, xxx xxxxxxx xxxxxxxxx xxxx&xxxx;Xxxxxxx xxxxxxxxxx xxxxx&xxxx;xxxx země xxxx jím xxxxxxxx xxxxxxx.
Xxxxxxxxxxxx významných Akcí (Major Xxxxx Xxxxxxxxxxxxx): Kontinentální xxxxxxxx&xxxx;Xxxxxxxxx olympijských výborů a xxxxxx xxxxxxxxxxxxx xxxxxxxxxx xxx xxxx sportů, xxxxx xxxxxx xxxx xxxxxx xxxxxx xxx xxxxxxxxxxxxx, xxxxxxxxxx xx xxxxx&xxxx;Xxxxxxxxxxx Akci.
Podání (Administration): Xxxxxxxxxxx, xxxxxxxx, xxxxxxxxx, xxxxxxxxxx xxxx xxxx xxxxxxxx xx xx&xxxx;Xxxxxxx&xxxx;xxxx&xxxx;Xxxxxx&xxxx;x&xxxx;Xxxxxxx&xxxx;xxxxx&xxxx;Xxxxxx, a xx&xxxx;Xxxxxxxx látky nebo Zakázané xxxxxx. Xxxx xxxxxxxx xxxx xxxxxxxxxx xxxxxxxxx se Zakázanou xxxxxx&xxxx;xxxx&xxxx;Xxxxxxxxx metodou uskutečněné xxxxxxxxxxxxx xxxxxxxxxx v xxxxx xxxx ("xxxx xxxx") xxx xxxxxxxx a xxxxxxx terapeutické xxxxx xx s jiným xxxxxxxxxxx zdůvodněním, a xxxx xxxxxxxxxx nakládání xx&xxxx;Xxxxxxxxxx xxxxxxx, které xxxxxx xxxxxxxx xxx&xxxx;Xxxxxxxxx Xxxx Xxxxxx, pokud xx x xxxxxxx xxxxxxx neprokáže, xx xxxx&xxxx;Xxxxxxxx xxxxx&xxxx;xxxxxx xxxxxx xxx skutečné x xxxxxxx xxxxxxxxxxxx xxxxx xxxx xxxx určeny xx xxxxxxx xxxxxxxxxxx xxxxxx.
Xxxxx&xxxx;(Xxxxxxx): Xxxxxxx jednání, xxxxx xxxxxxxxxxx významný xxxx x xxxxxxx xxxxxxx xxxxxxxxxxx plánovaně x porušení xxxxxxxxxxxxxxx xxxxxxxx. X porušení xxxxxxxxxxxxxxx pravidla založenému xxxxx xx&xxxx;Xxxxxx&xxxx;x xxxxxxxx xxxx xxxxxxx, pokud Osoba od Pokusu ustoupí xxxxx, xxx byl xxxxxxx třetí xxxxxxx, xxxxx se Pokusu neúčastní.
Pozitivní laboratorní xxxxx&xxxx;(Xxxxxxx Xxxxxxxxxx Finding): Xxxxxx x laboratoře xxxxxxxxxxxx&xxxx;XXXX&xxxx;xxxx xxxx laboratoře xxxxxxxxx&xxxx;XXXX, xxxxx xx xxxxx x&xxxx;Xxxxxxxxxxxx standardem pro xxxxxxxxxx identifikuje xx&xxxx;Xxxxxx&xxxx;xxxxxxxxxx&xxxx;Xxxxxxxx xxxxx&xxxx;xxxx xxxxxx&xxxx;Xxxxxxxxxx&xxxx;xx&xxxx;Xxxxxxx, xxxx xxxxx xxxxxxxxx na Použití Xxxxxxxx xxxxxx.
Xxx Xxxxxxx&xxxx;(Xx-Xxxxxxxxxxx): Xxxxxx xx 23:59 x xxx před Soutěží, xxxxx xx xx&xxxx;Xxxxxxxxx&xxxx;xxxxxxxxx, xx konce Soutěže a procesu xxxxxx&xxxx;Xxxxxx, xxxx je xx&xxxx;Xxxxxxx&xxxx;xxxxxx.&xxxx;XXXX&xxxx;xxxx xxx xxxxxxxxx xxxxx schválit xxxxxxxxxxxx xxxxxxxx, jestliže Mezinárodní xxxxxxxx&xxxx;xxxxxxxx xxxxxxxxxxx xxxxxxxxxx, xx xxxx xxxxxxxx xx xxx její xxxxx xxxxxxxx. Xx xxxxxxx xxxxxxxxx&xxxx;XXXX&xxxx;xxxxx podle alternativní xxxxxxxx xxxxxxxxxx xxxxxxx&xxxx;Xxxxxxxxxxxx xxxxxxxxxx Xxxx&xxxx;x konkrétním xxxxxx.
[Xxxxxxxx x Při Xxxxxxx: Mít všeobecně xxxxxxxxxx definici termínu Xxx xxxxxxx xxxxxxxxx xxxxx xxxxxxxxxxx mezi Xxxxxxxxx xx všech xxxxxxxx, xxxxxxxxx xxxx xxxxxxx nejasnosti xxxx Xxxxxxxxx xxxxxxx xxxxxxxxxxx xxxxxxxx xxxxx xxx Xxxxxxxxx Xxx xxxxxxx, xxxxxxxxx xxxxxxxxxx Xxxxxxxxxx xxxxxxxxxxx nálezům xxxx Xxxxxxxxx xxxxx Xxxx x pomáhá xxxxxxxxxx xxxxxxx xxxxxxxxxxxxx xxxxx xxxxxxxxx výkonu ze Xxxxxxxxxx látek Xxxx xxxxxx xx období Xxxxxxx.]
Xxxxxxx&xxxx;(Xxx): Užití, xxxxxxxx, xxxxxx, injekce, xxxx xxxxxxxxx jakýmkoli způsobem Zakázané xxxxx&xxxx;xxxx&xxxx;Xxxxxxxx metody.
Rekreační Xxxxxxxxx&xxxx;(Xxxxxxxxxxxx Xxxxxxx): Fyzická Osoba, xxxxxx xxxxxxxx xxxxxxxxx&xxxx;Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx; xx předpokladu, xx xxxxxx nebude xxxxxxxxx xxxxxx&xxxx;Xxxxx, xxxxx xxxxx pěti xxx xxxx xxxxxxxxx xxxxxxxxxx xxxxxxxx antidopingových pravidel xxxx&xxxx;Xxxxxxxxxx Xxxxxxxxxxx úrovně (jak xx xxxxxxxxxx každou Xxxxxxxxxxx federací x xxxxxxx s Mezinárodním standardem pro Testování a Xxxxxxxxxxx) nebo Sportovcem Xxxxxxx xxxxxx&xxxx;(xxx xxx definuje xxxxx&xxxx;Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx&xxxx;x xxxxxxx s Mezinárodním xxxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxx&xxxx;x Xxxxxxxxxxx), xxxxx zastupovala xxxxxxxxx zemi xx&xxxx;Xxxxxxxxxxx Xxxx&xxxx;x otevřené kategorii xxxx která byla xxxxxxxx xx kteréhokoli Registru xxx Testování či xxxxxx xxxxxxxx xxxxxxxxx x xxxxx xxxxxx xxxxxxxx xxxxxxxxx Xxxxxxxxxxx xxxxxxxx xxxx&xxxx;Xxxxxxx xxxxxxxxxxxxxx xxxxxxxxxx.
[Xxxxxxxx x Xxxxxxxxxxx Xxxxxxxxx: Xxxxxx "xxxxxxxx xxxxxxxxx" xx vyloučit xxxxxx, xxxxx xx xxxxxxx xx juniorské xxxx xxxxxx kategorie.]
Seznam (Prohibited Xxxx):&xxxx;Xxxxxx xxxxxxxxxxxxxx&xxxx;Xxxxxxxx látky a Zakázané xxxxxx.
Xxxxxx&xxxx;(Xxxxxxxxxxx): Xxxxxxxxxx xxxxx, xxxxx, xxx nebo samostatné xxxxxxxxx utkání. Xxxxxxxxx xxxxxxxxxxxx xxxxx xxxx xxxxxxxxxx xxxxxx běhu xx 100 xxxxx x xxxxxxxx. X xxxxxxxxx xxxxxx x xxxxxx xxxxxxxxxxx závodů, xxx se ceny xxxxxxx denně nebo x xxxxxx intervalech, xx rozdíl xxxx&xxxx;Xxxxxxx&xxxx;x&xxxx;Xxxx&xxxx;xxxxxx xxxxx xxxxxxxx xxxxxxxxx Xxxxxxxxxxx xxxxxxxx.
Xxxxxxxxx&xxxx;(Xxxxxxx): Jakákoli Osoba, xxxxx xxxxxxx xx xxxxxx xx mezinárodní xxxxxx (xxxxxx definují xxxxxxxxxx Xxxxxxxxxxx xxxxxxxx) xxxx na xxxxxxx xxxxxx (xxxxxx definují xxxxxxxxxx&xxxx;Xxxxxxx antidopingové xxxxxxxxxx).&xxxx;Xxxxxxxxxxxxx xxxxxxxxxx&xxxx;xx sama xxxx xxxxxxxxxx xxx aplikaci xxxxxxxxxxxxxxx pravidel xx&xxxx;Xxxxxxxxx, xxxxx xxxx Sportovcem xxxxxxxxxxx xxxxxx ani Sportovcem xxxxxxx úrovně, x xxx xx xxxx xxxxxxxx xxxxxxxx "Xxxxxxxxx". Xxx&xxxx;Xxxxxxxxx, kteří nejsou Sportovci xxxxxxxxxxx úrovně ani Sportovci národní xxxxxx, xxxx&xxxx;Xxxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxxxxxx xxxxxxx Xxxxxxxxx&xxxx;xxxx neprovádět xxxxx&xxxx;Xxxxxxxxx; neanalyzovat Vzorky pro xxxxx xxxxx&xxxx;Xxxxxxxxxx xxxxx; xxxxxxxxx xxxxxxx xxxxxxxxx o xxxxx xxxxxx xxxx xxxxxxxxxxx xxxxx; xxxxxxxxxxx xxxxxx&xxxx;XX. Avšak pokud xxxxx podle xxxxxx 2.1, 2.3 xxxx 2.5 k porušení xxxxxxxxxxxxxxx xxxxxxxx&xxxx;Xxxxxxxxxx, u xxxxx se Antidopingová organizace rozhodla, xx xxxxxxx xxxx xxxxxxxx xxxxxxxx, x xxxxx xxxxxxx pod xxxxxxxxxxx xxxx národní xxxxxx, xxx xxxx xxx xxxxxxxxx&xxxx;Xxxxxxxx&xxxx;xxxxxxxxx x&xxxx;Xxxxxx. Xxx xxxxx článků 2.8 x 2.9 x xxxxx xxxxxxxxxxxxx xxxxxxxxxxxxxx x&xxxx;Xxxxxxxxxx, xxxxx&xxxx;Xxxxx, xxxxx xxxxxxx xx xx xxxxxxx&xxxx;Xxxxxxx&xxxx;xx xxxxxx, xxxxx xxxxx xx xxxxxxxxx xxxxxxxxxxx&xxxx;Xxxxxxxxx, xxxxx xxxx jiné sportovní xxxxxxxxxx, která xxxxxxx&xxxx;Xxxxx, xx považována xx&xxxx;Xxxxxxxxx.
[Xxxxxxxx xx Xxxxxxxxx: Jednotlivci, xxxxx se xxxxxxx xxxxxx, mohou xxxxxx xx xxxxx z xxxx xxxxxxxxx: 1)&xxxx;Xxxxxxxxx xxxxxxxxxxx xxxxxx, 2) Xxxxxxxxx národní úrovně, 3) xxxxxxxxxxx, xxxxx xxxxxx Xxxxxxxxx xxxxxxxxxxx xxxx xxxxxxx xxxxxx, xxx xxx xxxxx xx xxxxxxxx xxxxxxxxx xxxxxxxx Mezinárodní xxxxxxxx xxxx Národní antidopingová xxxxxxxxxx, 4) Xxxxxxxxx Xxxxxxxxx a 5) xxxxxxxxxxx, xxx xxxxx xxxxx Xxxxxxxxxxx xxxxxxxx xxx Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx nevykonává pravomoc xxx xx xxxxxxxxxx xxxxxxxx xxxxxxxxx. Xx xxxxxxx Sportovce xxxxxxxxxxx x xxxxxxx xxxxxx xx xxxxxxxx antidopingová xxxxxxxx Kodexu s xxxxxxxx definicemi xxxxxx xxxxxxxxxxx a národní xxxxxx, xxxxx xxxxx xxxxxxxxx x xxxxxxxxxxxxxxx xxxxxxxxxx Xxxxxxxxxxxxx xxxxxxxx x Národních xxxxxxxxxxxxxxx xxxxxxxxxx.]
Xxxxxxxxx xxxxxxxxxxx xxxxxx&xxxx;(Xxxxxxxxxxxxx-Xxxxx Xxxxxxx):&xxxx;Xxxxxxxxx, xxxxx se xxxxxxx xxxxxx na xxxxxxxxxxx xxxxxx, xxx xx definována xxxxxx Xxxxxxxxxxx federací xx xxxxx x&xxxx;Xxxxxxxxxxxx xxxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxx&xxxx;x&xxxx;Xxxxxxxxxxx.
[Xxxxxxxx xx Xxxxxxxxx xxxxxxxxxxx xxxxxx: X xxxxxxx x Xxxxxxxxxxxx xxxxxxxxxx xxx Xxxxxxxxx a Xxxxxxxxxxx může Xxxxxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx, xxxxx xxxxxxx xx xxxxxxxxxxx Xxxxxxxxx xxxx Xxxxxxxxx mezinárodní xxxxxx, xxxx. podle xxxxxxxxx, účastí xx xxxxxxxxxxx Xxxxxxxxxxxxx xxxxxx, xxxxx typu xxxxxxx xxx. Musí xxxx xxxx xxxxxxxx xxxxxxxxx xxxxxx x xxxxxxxx xxxxxx, xxx Sportovci xxxxx xxxxxx x xxxxxx xxxxxxx, xxx xxxxx klasifikováni xxxx Xxxxxxxxx xxxxxxxxxxx úrovně. Xxxxx xxxxxxxxx xxxxxxxx xxxxxxxx xxxxx na xxxxxxxx Xxxxxxxxxxxxx xxxxxx, xxxx Xxxxxxxxxxx federace Xxxxxx xxxxxx Xxxxxxxxxxxxx xxxx xxxxxxxxx.]
Xxxxxxxxx národní xxxxxx&xxxx;(Xxxxxxxx-Xxxxx Xxxxxxx):&xxxx;Xxxxxxxxx, xxxxx xx xxxxxxx xxxxxx xx xxxxxxx xxxxxx, xxx xx definována xxxxxx&xxxx;Xxxxxxx xxxxxxxxxxxxxx xxxxxxxxxx&xxxx;xx xxxxx x&xxxx;Xxxxxxxxxxxx standardem pro Testování a Vyšetřování.
Terapeutická xxxxxxx&xxxx;("XX") (Xxxxxxxxxxx Xxx Xxxxxxxxx):&xxxx;Xxxxxxxxxxxx xxxxxxx&xxxx;xxxxxxxx&xxxx;Xxxxxxxxx&xxxx;xx zdravotním xxxxxxxxxxx Xxxxxx&xxxx;Xxxxxxxxx xxxxx&xxxx;xxxx&xxxx;Xxxxxxxxx xxxxxx, xxx xxxxx xxxxx, jsou-li splněny xxxxxxxx xxxxxxxxx x xxxxxx 4.4 x x&xxxx;Xxxxxxxxxxxx standardu pro Terapeutické výjimky.
Testování (Testing): Xxxxx xxxxxxx&xxxx;Xxxxxxxxx xxxxxxxx&xxxx;xxxxxxxxxx xxxxxxxxx xxxxx, xxxxx&xxxx;Xxxxxx, xxxxxxxxx xx&xxxx;Xxxxxxx&xxxx;x xxxxxxxxx&xxxx;Xxxxxx&xxxx;xx xxxxxxxxxx.
Xxxxxxxxxx&xxxx;(Xxxxxxxxx): Xxxxxxxxxx proces, xxxxx xxxxxx xx xxxxxxx xxxxxxx x xxxxxxxx xxxxxx, xxxxx xxxxxxxx x xxxxxx xxxxxxxxxxx ducha x xxxxxxxxxxx úmyslnému i xxxxxxxxxxx dopingu.
Vzorek (Sample xx Xxxxxxxx): Xxxxxxxx xxxxxxxxxx xxxxxxxx xxxxxxxx xx xxxxxx&xxxx;Xxxxxxxxx xxxxxxxx.
[Xxxxxxxx xx Xxxxxx: Někdy se xxxxx, xx odběr Xxxxxx xxxx porušuje xxxxxxxx xxxxxxxx xxxxxxxxxxxx xxxx xxxxxxxxxx xxxxxx. Xxxx xxxxxxxxx, že xxx takové xxxxxxx xxxxxxxxxx xxxxx základ.]
WADA (The Xxxxx Xxxx-Xxxxxx Agency): Xxxxxxx xxxxxxxxxxxxx xxxxxxxx.
Xxxxxxxx xxxxx&xxxx;(Xxxxxxxxxx Xxxxxxxxx): Xxxxxxxx xxxxx xxxx xxxxxxx xxxxx uvedená x&xxxx;Xxxxxxx.
Xxxxxxxx xxxxxx&xxxx;(Xxxxxxxxxx Xxxxxx): Xxxxxxxx xxxxxx xxxxxxx x&xxxx;Xxxxxxx.
3.2&xxxx;Xxxxxxxxxx xxxxx x&xxxx;Xxxxxxxxxxxxx xxxxxxxxx&xxxx;xxx xxxxxxx soukromí a xxxxxxxx údajů
Osobní xxxxx:&xxxx;Xxxxx, xxxx jiné včetně Citlivých xxxxxxxx údajů, týkající xx&xxxx;Xxxxxxxxx&xxxx;xxxx xxxx&xxxx;Xxxxx, xxxxxxx xxxxxxxxx xxxx prokázána xxxx xx možné xx prokázat, x xxxxxxx xxxxx xxxx&xxxx;Xxxxxxxxxxxx&xxxx;xxxxxxxx x souvislosti x&xxxx;xxxxxxxxxxxxxx xxxxxxxx Xxxxxxxxxxxxx organizace.
[Poznámka x&xxxx;Xxxxxxx xxxxxx:&xxxx;Xxxxxx se, xx&xxxx;Xxxxxx xxxxx&xxxx;xxxxxxxx xxxx xxxx xxxxx xxxxxxxx xx xxxxx Sportovce, xxxx xxxxxxxx, kontaktních xxxxx a xxxxxxxxxxx xxxxxxxx, xxxxx xxxxxx, xxxxxxxxxxx TV (xxxx-xx xxxxxx), xxxxxxxx xxxxxxxxxxxxxxx xxxxx x výsledků xxxxxxxx xxxxxx (xxxxxx xxxxxxxxxxxxxxx xxxxxx, xxxxxxxx x xxxxxx).&xxxx;Xxxxxx xxxxx&xxxx;xxxx xxxxxxxx xxxxxx x xxxxxxxxx xxxxxxxxx týkající xx xxxxxx Xxxx, xxxx například xxxxxxxxxxxxxx xxxxxxxxx xxxxxxxxxx xxxx xxxxxx Xxxx, které xxxxxxx xx Xxxxxxxxxx, xxxxxxxx xxx xxxx xx pomáhají x xxxxx Xxxxxxxxxxxxx xxxxxxxx. Xxxxxx informace xxxx xxxxx xxxxxxxxxx xx&xxxx;Xxxxxx xxxxx&xxxx;x xxxx tímto Xxxxxxxxxxxx xxxxxxxxxx regulovány xx xxxxx xxxx xxxxxx&xxxx;Xxxxxxxxxx, xxx xxxxxx xx xx, xxx xx xxxxxxxxx xxxxxxxxxxx xxxxx zapojen xx xxxxxxxxxxxxxx xxxxxx.]
Xxxxxxxxxx&xxxx;(x xxxxx xxxxx&xxxx;Xxxxxxxxxx,&xxxx;Xxxxxxxxxxxx&xxxx;xxxx.): Xxxxxxxxxxxxx, přístup, xxxxxxxxxx, xxxxxxxx, xxxxxxxxx, xxxxxxxxxxxx, přenos, xxxxx, xxxxxx xxxx jiné xxxxxxxxx&xxxx;Xxxxxxxx xxxxx.
3.3&xxxx;Xxxxxxxxxx xxxxx, xxxxx xxxx xxxxxxxxxx xxx&xxxx;Xxxxxxxxxxx xxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx xxxxxxx
Xxxxxx xxx xxxxxxxxxxxx xxxxxxx ("XXX")&xxxx;(Xxxxxxxxxxx Xxx Xxxxxxxxx Xxxxxxxxx xx "XXXX"):&xxxx;Xxxxx xxxxxxxxxx&xxxx;Xxxxxxxxxxxxxx xxxxxxxxxx&xxxx;xx účelem xxxxxxxxxx žádostí x&xxxx;XX.
XXX XXXX&xxxx;(XXXX XXXX): Xxxxx xxxxxxxxxx&xxxx;XXXX&xxxx;xx xxxxxx xxxxxxxxxxxxx xxxxxxxxxx xxxxxx&xxxx;Xxxxxxxxxxxxxxx organizací o TV.
Terapeutický (Therapeutic): Xxxxxxxxxx xx x xxxxx zdravotního xxxxx xxxxxxxxx xxxxxxxxxx xxxx xxxxxxxx; nebo poskytující xx napomáhající uzdravení.
3.4 Výklad xxxxx
3.4.1&xxxx;Xxxxxxxxx xxxx&xxxx;Xxxxxxxxxxxxx xxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx xxxxxxx&xxxx;xxxx xxxxxxxxxx x xxxxxxxxxx x francouzštině. X případě jakýchkoli xxxxxxx mezi xxxxxxxxx x xxxxxxxxxxxx xxxxx xxxxx anglická xxxxx.
3.4.2&xxxx;Xxxxxx xxxx&xxxx;Xxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxx standard pro Terapeutické výjimky vypracován x ohledem na xxxxxx xxxxxxxxxxxxxxx, lidská xxxxx x další xxxxxxxxx xxxxxx xxxxxx. X x tomto xxxxxx xxxx xxxx xxxxxxxx x xxxxxxxxx.
3.4.3&xxxx;Xxxxxxxx xxxxxxxxxxx různá ustanovení Mezinárodního xxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx xxxxxxx&xxxx;xxxx použity x xxxxxx xxxxxxxxxxxx.
3.4.4&xxxx;Xxxxx xxxx stanoveno xxxxx, xxxxxx xx části x xxxxxx xxxx xxxxxx xx xxxxx x xxxxxx&xxxx;Xxxxxxxxxxxxx xxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx xxxxxxx.
3.4.5&xxxx;Xxxxxx "xxx" xxxxxxx x&xxxx;Xxxxxxxxxxxx xxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx xxxxxxx&xxxx;xxxxxxxxxx xxx, xxxxx xxxx xxxxxxx jinak.
3.4.6 Přílohy Mezinárodního xxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx xxxxxxx&xxxx;xxxx stejné xxxxxxx xxxxxxxx xxxx xxxxxx&xxxx;Xxxxxxxxxxxxx xxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx xxxxxxx.
XXXXX XXXX: XXXXXXXXX A POSTUP XXX XXXXXXXXX TV
4.0 Udělování TV
Sportovec, xxxxx x&xxxx;Xxxxxxxxxxxxxx&xxxx;xxxxxx potřebuje xxxxxx&xxxx;Xxxxxxxxx látku nebo Zakázanou xxxxxx, xxxx xxxx&xxxx;Xxxxxxxx&xxxx;xxxx&xxxx;Xxxxxxx zakázané xxxxx xxxx metody xxxxxxx o TV, pokud xxxx&xxxx;Xxxxxxxxx&xxxx;xxxxxxxx xxxxxxx x&xxxx;XX&xxxx;xx xxxxxxx platností xxxxx xxxxxx 4.1. X xxxx xxxxxxxxx xxxx xxx xxxxxxx xxxxxxxx xxxxxx 4.2.
[Poznámka x xxxxxx 4.0: Xxxxx xxxxxx situace, xxx xx Xxxxxxxxx nemocný x užívá nebo xx x držení Xxxxxxxxx xxxxx xxxx Xxxxxxxxx xxxxxx xxxxxxx, xxx se na xxxx začnou xxxxxxxxx xxxxxxxxxxxxx pravidla. X xxxxxxx xxxxxxx xxxxxxxxxx xxxxxx předchozí užívání/držení XX x xxxx xxxxxxxxxx&xxxx;XX&xxxx;x xxxxxxx platností.]
4.1 Na xxxxxxx&xxxx;XX&xxxx;xx xxxxxxx xxxxxxxxx xxxx&xxxx;Xxxxxxxxx&xxxx;xxxxxxx x&xxxx;XX&xxxx;xxx&xxxx;Xxxxxxxxx látku nebo Zakázanou xxxxxx&xxxx;xxxx, co xxxxxxxx xxxxx nebo xxxxxx&xxxx;xxxx&xxxx;xxxx&xxxx;xxx x xxxxxx.
Xxxxxxxxx&xxxx;xxxx o TV požádat xx xxxxxxx xxxxxxxxx (xxxx xxxx xxxxx xxxxxxxx xxxxxxxx xxxxxx 4.2), xxxxx xxxxx xxxxxxx x xxxxxxxxxxxxx xxxxxxx:
x)&xxxx;Xxxxxxx xxxxxxx xxxxxxxxxx xxxxx nebo xxxxx xxxxxxxx zdravotního xxxxx;
x)&xxxx;Xxxxx xxxxxxxxxx xxxxxxxxxx xxxxx xxxxxxxx xxxx xx xxxxxxxxxxxx, aby Sportovec podal žádost x&xxxx;XX&xxxx;(xxxx XXX xxxxxxx xxxxxx) před xxxxxxx&xxxx;Xxxxxx;
x)&xxxx;X xxxxxx xxxxxxxxxxxxxxx xxxxxxxx xxxxxx xxxx disciplín xx národní xxxxxx&xxxx;Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx Sportovce nepovolila xxx xxxxxxxxxxxx, aby Sportovec požádal x potenciální TV (viz xxxxxxxx x xxxxxx 5.1);
x)&xxxx;Xxxxx xx&xxxx;Xxxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxxxxxx xxxxxxx&xxxx;Xxxxxx&xxxx;xx&xxxx;Xxxxxxxxx, xxxxx xxxx&xxxx;Xxxxxxxxxx xxxxxxxxxxx xxxxxx&xxxx;xxxx&xxxx;Xxxxxxxxxx národní xxxxxx, x xxxxx&xxxx;Xxxxxxxxx&xxxx;x&xxxx;Xxxxxxxxxxxxxx&xxxx;xxxxxx používá Zakázanou xxxxx&xxxx;xxxx&xxxx;Xxxxxxxxx xxxxxx,&xxxx;Xxxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxx xxxxxxx&xxxx;Xxxxxxxxx&xxxx;xxxxxxx o TV se xxxxxxx xxxxxxxxx; xxxx
x)&xxxx;Xxxxxxxxx Xxxxxx Xxxx Xxxxxx&xxxx;x&xxxx;Xxxxxxxxxxxxxx&xxxx;xxxxxx&xxxx;Xxxxxxxxx látku, xxxxx xx xxxxxxxx xxxxx&xxxx;Xxx Soutěži.
[Poznámka x xxxxxx 4.1: Xxxxxxx xxxxx z xxxxxxx xxx TV xx xxxxxxx xxxxxxxxx xxxxxxxxx, xx bude XX xxxxxxxx xxxxxxx; xxxxxxx xx, že žádost Xxxxxxxxx xxxx xxx xxxxxxxxxxx xxxxx xxxxxx 4.2, aby xx xxxxxxxx, zda xxxx xxxxxxx stanovené podmínky XX.]
[Xxxxxxxx x xxxxxx 4.1 c), d) x e): X xxxxxxx, xxx je xx odběru Xxxxxx xxxxx žádost o XX xx xxxxxxx xxxxxxxxx, xx Xxxxxxxxxx xxxxxxx doporučuje, xxx xx xxxxxxx xxxxxxxxx xxxxxxxxx xxxxxxxxxxx a xxxx xxxxxxxxxx xxxxxxxx xxxxxxx podmínek xxx XX stanovených v xxxxxx 4.2.]
[Xxxxxxxx x xxxxxx 4.1 x): Xxxxx xx xxxxx xxxxxxx, kdy Sportovec x&xxxx;Xxxxxxxxxxxxxx&xxxx;xxxxxx Xxxxxxx Mimo Xxxxxx xxxxx, která xx xxxxxxxx pouze Xxx Soutěži, xxx xxxxxxxx riziko, že xxxxx xxxxxxx Při Xxxxxxx v xxxxxx xxxx.
X takových situacích xxxx Antidopingová xxxxxxxxxx xxxxxxx Xxxxxxxxx xxxxxxx x TV xx xxxxxxx xxxxxxxxx (xxxxx Xxxxxxxxx xxxxxxxxx o XX xxxxxx). Toto xxxx xxxxxxx x xx, xxx Antidopingové xxxxxxxxxx nemusely xxxxxx xxxxxxxxx žádosti x XX, které xxxxxx xxx xxxxx.]
4.2&xxxx;Xxxxxxxxx&xxxx;xxxx xxx xxxxxxx&xxxx;XX, pokud (x xxxxx xxxxx) může xxxxxxxx, xxx xxxxxxx xxxxxxxxxxxxxxxx, xx xx xxxxxxx xxxxx z xxxxxxxxxxxxx podmínek:
a) Daná Zakázaná xxxxx&xxxx;xxxx&xxxx;Xxxxxxxx xxxxxx&xxxx;xx nutná k xxxxx xxxxxxxxxxxxxxxxx xxxxxxxxxxx xxxxxx xxxxxxxxxxx xxxxxxxxxxx xxxxxxxxxx xxxxxx.
[Xxxxxxxx k xxxxxx 4.2 a): Xxxxxxx Xxxxxxxx látky xxxx Xxxxxxxx xxxxxx xxxx xxx xxxxx xxxxxxxx nezbytného xxxxxxxxxxxxxx xxxxxxxxx xxx samotnou xxxxxx.]
x)&xxxx;Xxxxxxxxxxxx&xxxx;Xxxxxxx Xxxxxxxx látky nebo Zakázané xxxxxx&xxxx;x xxxxxxxx pravděpodobností xxxxxxxxx xxxxx xxxx xxxxxxx xxxxxx&xxxx;Xxxxxxxxx, xxx xxxx xxx očekávat xxxxxxxxxx xx xxxxxxxxxx xxxxxxxxxxx xxxxx xxxxxxxx xx xxxxx xxxxxxxxxxx xxxxxx.
[Xxxxxxxx k xxxxxx 4.2 x): Xxxxxxxx xxxxxxxxx xxxx Xxxxxxxxx xxxx třeba xxxxx xxxxxxxxxxxx. Normální xxxxxxxxx xxxx xxx konkrétního Xxxxxxxxx xx xxxx xxxxxxxxx xxxx, xxx xxx zdravotních xxxxxxxx, xxxxx kterým Sportovec xxxx x XX.]
x)&xxxx;Xxxxxxxx xxxxx&xxxx;xxxx&xxxx;Xxxxxxxx xxxxxx&xxxx;xx xxxxxxxxxxx xxxxxx zdravotních potíží x neexistuje žádná xxxxxxxxxx xxxxxxxx&xxxx;Xxxxxxxxxxxx&xxxx;xxxxxxxxxxx.
[Xxxxxxxx x xxxxxx 4.2 c): Xxxxx musí vysvětlit, xxxx xxxx xxxxxxx xxxxx xxxxxxxxxxxx, xxxx. xx základě zkušeností, xxxxxxx xxxxxxxxxx xxxxxx xxxx xxxxxx xxxxxxxxxx xxxxxxxxxx, xxxxxx, xx-xx xx xxxxxxxxxx, xxxxxxxxxxx xxxxxxxxxx lékařské xxxxx x xxxxxxxx xxxxxxxx x xxxxx. Xxxx xxxx xxxx xxxxx xxxxxxxxx xxxxxxxxx alternativy xxxx xxxxxxxx Zakázané xxxxx xxxx Zakázané xxxxxx.]
x)&xxxx;Xxxxxxx xxxxxxx&xxxx;Xxxxxxxx xxxxx&xxxx;xxxx&xxxx;Xxxxxxxx xxxxxx&xxxx;xxxxxx xxx následkem, xxxxx xxxx xxxxxx, xxxxxxxxxx&xxxx;Xxxxxxx&xxxx;(xxx&xxxx;XX) látky nebo xxxxxx, xxxxx xxxx x xxxx xxxxxxxx&xxxx;Xxxxxxx&xxxx;xxxxxxxx.
[Xxxxxxxx x xxxxxx 4.2: Xxxxxxxxx XXXX s xxxxxx "Lékařské informace xxx xxxxxxxxxxx x XX", umístěné na xxxxxxxx stránkách WADA, xx xx xxxx xxxxxxxx, xxx xx xxxxxxxxx xxxxxxxxxx těchto xxxxxxxx, xxxxx jde x xxxxxxxxx xxxxxxxxx xxxxxx.
Xxxxxxx XX xx xxxxxxxx pouze xx xxxxxxx podmínek xxxxxxxxxxx x článku 4.2. Xxxxxxxxxxxx, zda xx Xxxxxxxx xxxxx nebo Xxxxxxxx xxxxxx klinicky xxxxxxxxxxxx xxxx xxxxxxxxxxxxxx, xxxx zda xx xxxx Xxxxxxx xxxxxxx xx xxxxx jurisdikcích.
Pokud XXX Xxxxxxxxxxx federace xxxx Organizátora významné Xxxx xxxxxxxxx, xxx xxxxx nebo xxxxxxx XX xxxxxxxxxx jinou Xxxxxxxxxxxxxx xxxxxxxxxx (xxx xxxxxx 7), a xxxxx XXXX xxxxxxxxxxx xxxxxxxxxx x xxxxxxx (xxxx neudělení) XX (xxx článek 8), xxxxxxxx xxxx xxxxxx xxxx x případě xxxxxxxxxx xxxxxxx x Xxxxxxxxxxxxx xxxxxxx Komisí xxx XX xxxxx xxxxxx 6, xx., xxxxxxxx Xxxxxxxxx při xxxxxxx pravděpodobnosti, xx xx xxxxxxx každá x podmínek článku 4.2?]
4.3&xxxx;Xx xxxxxxxxxxx okolností x bez ohledu xx xxxxxxxx xxxx xxxxxxxxxx tohoto Mezinárodního xxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxxxxx xxxxxxx&xxxx;xxxx&xxxx;Xxxxxxxxx&xxxx;xxxxxxx x xxxxxxxxx&xxxx;Xxxxxxxxxxxxxx&xxxx;Xxxxxxx Xxxxxxxx xxxxx&xxxx;xxxx&xxxx;Xxxxxxxx metody se xxxxxxx xxxxxxxxx, xxxxx xx xxxx xxxxxxx, xxxxx by x xxxxxxx na xxxx Xxxxxx xxxx xxxxxx xxxxxxxxxxxxx&xxxx;XX&xxxx;xx xxxxxxx xxxxxxxxx xxxxxxxx. X&xxxx;Xxxxxxxxx xxxxxxxxxxx xxxxxx&xxxx;x&xxxx;Xxxxxxxxx národní xxxxxx xxxx&xxxx;Xxxxxxxxxxxxx organizace vyhovět xxxxxxx x&xxxx;XX&xxxx;xx zpětnou xxxxxxxxx xxxxx xxxxxx xxxxxx xxxxx x xxxxxxxxxx xxxxxxxxx&xxxx;XXXX&xxxx;(x&xxxx;XXXX&xxxx;xxxx podle svého xxxxxxxxxxx xxxxxxx s xxxxxxxxxxx&xxxx;Xxxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxxxxxxx, nebo xxx zamítnout).
U Sportovců, kteří xxxxxx&xxxx;Xxxxxxxxx mezinárodní xxxxxx&xxxx;xxxx&xxxx;Xxxxxxxxx xxxxxxx úrovně, xxxx xxxxxxxxx&xxxx;Xxxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxxxxx xxxxxxx x&xxxx;XX&xxxx;xx xxxxxxx xxxxxxxxx xxxxx xxxxxx xxxxxx xxx xxxxxxxxx konzultace x&xxxx;XXXX;&xxxx;XXXX&xxxx;xxxx xxxx xxxxxxx xxxxxxxxxx&xxxx;Xxxxxxxxxxxxx organizace udělit TV se zpětnou xxxxxxxxx xxxxx xxxxxx xxxxxx přezkoumat a xxxx podle svého xxxxxxxxxxx xxxxxxx x xxxxxxxxxxx xxxxxxxxx, nebo xxx xxxxxx. Xxxxx xxxxxxxxxx rozhodnutí učiněnému WADA a/nebo Antidopingovou xxxxxxxxxx&xxxx;xxxxx tohoto článku xxxxx xxxxxx xxxxxxx xxxxx xxxxxx x xxxxxxxx xxxxxxxxxxxxxxx xxxxxxxx xxx xxxxxxxxxxxxxxx xxxxxxxx, xxx jinak.
Veškerá rozhodnutí Antidopingové xxxxxxxxxx&xxxx;xxxxx tohoto xxxxxx 4.3, xx xx xxxxxxxxxx, xxxx xxxxxxxxx&xxxx;XX, xxxx být xxxxxxxxx xxxxxxxxxxxxxxx&xxxx;XXXXX&xxxx;x xxxxxxx x xxxxxxx 5.5.
[Xxxxxxxx x xxxxxx 4.3: Xxx xx předešlo pochybnostem, xxxx xxx podle xxxxxx 4.3 xxxxxxx xxxxxxxxx xx xxxxxxx xxxxxxxxx, i když xxxxxx xxxxxxx xxxxxxxx x článku 4.2 (xxxxxx xxxxxxx xxxxxx xxxxxxxx xxxx xxx xxxxxxxxxx xxxxxxxxxx). Xxxx xxxxx xxxxxxxxxx xxxxxxx xxxxx xxxxxx xxxx. xxxxxx, xxxx Xxxxxxxxx xxxxxxxxx x TV xxxxxx; zkušenosti Xxxxxxxxx; xxxxxxxx, xxxxx Xxxxxxxxx xxxxxxx získal; zda Xxxxxxxxx deklaroval Xxxxxxx xxxxx nebo metody xx formulář Xxxxxxxxx xxxxxxxx; x nedávné xxxxxxxx TV Sportovce. Xxx svém xxxxxxxxxxx xx xxxx WADA xxxxx svého xxxxxxx xxxxxxx xx členem (xxxxx)&xxxx;XXX WADA.]
5.0 Povinnosti Antidopingových xxxxxxxxxx&xxxx;xxxxxxxx xx&xxxx;XX
5.1&xxxx;Xxxxxx 4.4&xxxx;Xxxxxx&xxxx;xxxxxxxxxxx x) xxxxx&xxxx;Xxxxxxxxxxxxx organizace má pravomoc xxxxxxxxxx o TV; b) xxx by xxxx xxx tato xxxxxxxxxx x&xxxx;XX&xxxx;xxxxxxxx x xxxxxxxxxxxx xxxxxx&xxxx;Xxxxxxxxxxxxxxx xxxxxxxxxxxx; x x) xxx xxxxx xxx rozhodnutí x&xxxx;XX&xxxx;xxxxxxxxxxxxx x/xxxx xxx xx xxxxx xxx lze xxxxxxx.
[Xxxxxxxx k bodu 5.1: Xxx xxxxxxx 1 - xxxxxxxxx xxxxxxx shrnující xxxxxxxx xxxxxxxxxx xxxxxx 4.4 Xxxxxx.
X xxxxxxxxx, xxx xxxxxxxxx x xxxxxxxx xxxxxxx xxxxxxxx xxxxx Xxxxxxx antidopingovou xxxxxxxxxx x tomu, xxx xxxxxxxxxxxxxxx xxxxxx xxxxxx xxxx xxxxxxxxxx před xxxxxx xxx xxxxxxxxx Xxxxxxxxx (jak xx xxxxxxxxxxx dle článku 4.4.1 Mezinárodního xxxxxxxxx xxx Xxxxxxxxx x Xxxxxxxxxxx), Národní antidopingová xxxxxxxxxx xxxx odmítnout xxxxxxxxx xxxxxx xxxxxxx x TV Xxxxxxxxx x některých anebo xx xxxxx xxxxxxxxxxxxx xxxxxx nebo disciplín, xxx x tom xxxxxxx xxxx povolit xxxxxxx takovému Xxxxxxxxx, xxxxxxx je xxxxxxxx xxxxxxx Xxxxxx, xxxxx x XX xx xxxxxxx xxxxxxxxx. Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx xx xxxx, xx xxxxxxxxx Xxxxxxxxx, xxxxxxx se xx týká, xxxxx xxxxxx xxxxxxxxxx na xxxxx webových stránkách.
Článek 4.4.2 Kodexu specifikuje xxxxxxxxx Národní xxxxxxxxxxxxx xxxxxxxxxx xxxxxxxxxx o XX v xxxxxxx Xxxxxxxxx, kteří xxxxxx Xxxxxxxxx xxxxxxxxxxx úrovně. X xxxxxxx sporu xxxxxxx toho, xxxxx Xxxxxxx xxxxxxxxxxxxx organizace xx měla xxxxxxxxx xxxxxx x XX Xxxxxxxxx, jenž xxxx Xxxxxxxxxx xxxxxxxxxxx xxxxxx, xxxxxxxx XXXX. Rozhodnutí XXXX xxxx xxxxxxx x xxxxx se xxxxx xxxx odvolat.]
5.2 Pro xxxxxxxxx pochybností, když Národní xxxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxxx&xxxx;Xxxxxxxxx XX, xxxx&xxxx;XX&xxxx;xx xxxxxx xx xxxxxxx xxxxxx x xxxxxxxxx xxxxxxx a xxxxxx být xxxxxxxx xxxxxx jinými Národními antidopingovými xxxxxxxxxxxx&xxxx;xxxxx článku 7.0 (xxxx. xxxxx byla Sportovci udělena TV jeho Národní xxxxxxxxxxxxxx organizací a ten xxxx xxxxxxx nebo xxxxxxx v xxxx xxxx&xxxx;Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx, xxxx tato TV platná, pokud xxxx&xxxx;Xxxxxxxxx&xxxx;xxxxxxxx takovou xxxxx&xxxx;Xxxxxxx xxxxxxxxxxxxxx xxxxxxxxxx).
5.3&xxxx;Xxxxx&xxxx;Xxxxxxx antidopingová xxxxxxxxxx, Xxxxxxxxxxx federace xxxx&xxxx;Xxxxxxxxxxx xxxxxxxxxx xxxx&xxxx;xxxx xxxxxxxxx&xxxx;XXX,&xxxx;xxxxx xxxxxxxx, zda xxxxxxx o udělení xxxx xxxxxx&xxxx;XX&xxxx;xxxxxxx xxxxxxxx xxxx x článku 4.2.
[Xxxxxxxx k xxxxxx 5.3: Xxx xxxxxxxxx xxxxxxxxxxx, xxxxxxx xxxxxxxx xxxxxxxxxxx x xxxxxxxx 4.1 x 4.3 xxxx určit xxxxxxxxx Xxxxxxxxxxxxx xxxxxxxxxx xx xxxxxxxxxx se xxxxxx (xxxxx) XXX.
X xxxx xx Xxxxxxxxxxx xxxxxxxx xxxx xxxx zvolit, xx xxxx xxxxxxx xxx dříve udělené XX xxxxxxxxxxx, musí xxxxxxxxx mechanismus, xxxxxx Xxxxxxxxx účastnící se Xxxx xxxx moci xxxxxx xxxxx XX, xxxxxxxx xx bude xxxxxxx. Xx xx xxxxxx Organizátorovi xxxxxxxxxx xxxx, xxx xx xxxxxx xxx xxxxx xxxx svou xxxxxxx XXX, xxxx xxx xxxxxx tento xxxx xx schválení xxxxxxxx xx xxxxx xxxxx. Xxxxx by v xxxxxx případě xxxx xxx zajistit, xxx Xxxxxxxxx xxxxxxxxx se xxxxxx Xxxx xxxx xxxxxxx xxxxxx XX xxxxxx x x xxxxx účinností xxxx xxx, xxx začnou xxxxxxxx.]
x)&xxxx;XXX&xxxx;xx xx měly xxxxxxx xxxxxxx ze xxx (3) xxxxxx xx xxxxxxxxxxx x xxxx a léčbou Sportovců a xxxxxxxx xxxxxxxx xxxxxxxx x xxxxxxxxx zátěžové xxxxxxxx. V případech, xxx xx xxxxxxxxxx xxxxxxxx xxxxxxxxx (např. x&xxxx;Xxxxxxxxx&xxxx;x xxxxxxxxxx, xxx xx xxxxx xxxx xxxxxx týká xxxxxxxxx&xxxx;Xxxxxxxxx), xx alespoň xxxxx (1) z členů KTV měl xxx xxxxxx xxxxxxxxxx. Xxxxx (1) x xxxxx, který xx xxxxx, by xxx xxxxxxx xxxx předseda KTV.
b) Aby xxxx xxxxxxxxx nestrannost xxxxxxxxxx, xxxx xxxxxxx xxxxxxx&xxxx;XXX&xxxx;xxxxxxxx xxxxxxxxxx x xxxxxxxxx střetu zájmů x x zachování xxxxxxxxxx xxxxx (xxxx xxxxxxxxxx xx k xxxxxxxxx na xxxxxxxx xxxxxxxxx&xxxx;XXXX).
5.4&xxxx;Xxxxx&xxxx;Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx, Xxxxxxxxxxx xxxxxxxx nebo Organizátor xxxxxxxxxx Akcí musí xxxxxxxxx xxxxx xxxxxx, jak xxxxx x xxx&xxxx;XXX&xxxx;x&xxxx;XX, xxxxx je v xxxxxxx s xxxxxxxxx xxxxxx&xxxx;Xxxxxxxxxxxxx xxxxxxxxx. Xxxx xxxx zveřejnit xxxxxxxxxxx xxxxxx xxxxxxx xxx, xx (xxxxxxxxx) xxxxxx xxxx xxxxxxxxx xx xxxxx viditelném xxxxx xx xxxxx xxxxxxxx xxxxxxxxx.
5.5&xxxx;Xxxxx&xxxx;Xxxxxxx antidopingová xxxxxxxxxx, Xxxxxxxxxxx federace nebo Organizátor xxxxxxxxxx akcí musí xxxxxxxxxx xxxxxxx xxxxxxxx (x xxxxxxxxxx nebo francouzštině) x xxxxx xxxxxxxxxxxx xxxxx&xxxx;XXX&xxxx;x xxxxxxx xx xxxxxxxxx&xxxx;XX&xxxx;x x všech xxxxxxxxxxxx udělit či xxxxxxxxx xxxxxxx 7V xxxxxx Antidopingových xxxxxxxxxx xxxxxxxxxxxxxxx XXXXX, x xx co xxxxxxxx, x každém případě xx xxxxxxx xxxxx (21) xxx xx xxxxxxx rozhodnutí. Xxxxxxxxxx x zamítnutí TV musí obsahovat xxxxxxxxxx xxxxxx pro xxxxxxxxx. Ohledně xxxxxxxxx&xxxx;XX&xxxx;xxxx xxxxxxxx informace obsahovat (x angličtině xxxx xxxxxxxxxxxxx):
x)&xxxx;xxx byl Sportovec oprávněn xxxxxxx x&xxxx;XX&xxxx;xx xxxxxxx platností xxxxx xxxxxx 4.1 x xxxxxxxxxx xxxxxx xxxx, xxxx xxx xxx&xxxx;Xxxxxxxxx&xxxx;xxxxxxxx xxxxxxx x&xxxx;XX&xxxx;xx xxxxxxx platností, xxx xx xxxx xxxxxxx xxxxx xxxxxx 4.3 x xxxxxxxxxx důvodů xxxx;
x)&xxxx;xxxxxxxxxx xxxxx xxxx xxxxxx, dávkování, xxxxxxxxx, xxxxxxxx způsob Podávání, xxxx xxxxxx&xxxx;XX&xxxx;(x xxxx xxxxxxxxxx xxxxx, xxxxx se xxxx) x xxxxxxx xxxxx xxxxxxxx xxxxxxxxx x xxxxxxxxxxx x&xxxx;XX; x
x)&xxxx;xxxxxxxx žádosti o TV (pokud xxxx xxxxxxx elektronicky x XXXXX) x xxxxxxxxxx xxxxxxxxx xxxxxxxxxxx xxxxxxxxxxx, xx xxxxxxxx xxxxxx 4.2, xxxxx xxx o tuto TV, xxxx splněny (xxxx xxxxxxxxx budou x xxxxxxxxx xxxxx&xxxx;XXXX,&xxxx;Xxxxxxx antidopingové xxxxxxxxxx Sportovce, Xxxxxxxxxxx xxxxxxxx x&xxxx;Xxxxxxxxxxxxxx významných xxxx, xxxx xxxxxx&xxxx;Xxxx, xxxxx se Sportovec chce xxxxxxxxx).
[Xxxxxxxx x xxxxxx 5.5: Xxxxx xx používán xxxxxxxx xxxxxxx x XX, může xxx Xxxxxxxxxxxxxxx xxxxxxxxxxxx xxxxxxxx xx xxxxxx xxxxxx, xxx na xxxxxxxxx xxxx xxxxxx xxxxxxx xxxxxxxx nebo francouzský xxxx a xxxx xxx poskytnut anglický xxxx francouzský xxxxxxx xxxxxx.
Xxxx xxx xxxxxxxxxx xxxxx lékařské xxxxxxx, xxxxxx xxxxxxxxxxxxxx xxxxx, xxxxxxxxxxxxx xxxxxxxx x xxxxxx, xxx xxxxxx xxx přeloženy do xxxxxxxxxx xxxx francouzštiny. Xx systému XXXXX xxxx musí xxx xxxxxx xxxxxxxxx xxxxxxx xxxxx klíčových xxxxxxxxx (xxxxxx klíčových xxxxxxxxxxxxxx xxxxx) s dostatečnými xxxxxxxxxxx, xxx xxxx xxxxx jasně stanovit xxxxxxxx. Xxxxxxx xx xxxxxxxxxx, xxx xxxxxx xxxxxxx xxxxxxxxx xxxxx xxxx xxxx xxxxx x odpovídajícími xxxxxxxxxx xxxxxxxxx, xxx xxxx xxxxx xxxxxxxx xxxxxxxxx xxxxxxx xxxxxxxx a xxxxxxx. Xxxxxxxxxxx/xxxxxxxx překlady xxxx xxxxxxxxx příslušná Xxxxxxxxxxxxx xxxxxxxxxx xxxx XXXX, xx xxxxxxxx.]
5.6&xxxx;Xxxx&xxxx;Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxxx&xxxx;Xxxxxxxxx TV, xxxx xxx xxxxxxx xxxxxxxxx, xx x) xx&xxxx;XX&xxxx;xxxxxx pouze xx xxxxxxx xxxxxx a x) jestliže xx&xxxx;Xxxxxxxxx&xxxx;xxxxx&xxxx;Xxxxxxxxxx xxxxxxxxxxx xxxxxx&xxxx;xxxx xx xxxx xxxxxxxx&xxxx;Xxxxxxxxxxx akce, xxxxxx xxxx&xxxx;XX&xxxx;xxx xxxx xxxxx xxxxxx, xxxxx xxxxxx uznána xxxxxxxxxx Xxxxxxxxxxx federací nebo Organizátorem xxxxxxxx akce v xxxxxxx x xxxxxxx 7.0. Xxx xx&xxxx;Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxx xxxxxx&xxxx;Xxxxxxxxx&xxxx;xxxxx, xxx xxxxxxxxx xxxxx o xxxxxxx&xxxx;XX&xxxx;Xxxxxxxxxxx xxxxxxxx xxxx&xxxx;Xxxxxxxxxxxx xxxxxxxx Akce a xxxx xx jej xxxx x xxxxxxxxx xx xxxxx procesu xxxxxxxx&xxxx;XX.
5.7&xxxx;Xxxxx Xxxxxxxxxxx xxxxxxxx nebo Organizátor xxxxxxxx Xxxx&xxxx;xxxx xxxxx xxxxxxxx (xxxxxxxxx xxx, xx xxx xxxxxx xx xxxxx viditelném xxxxx xx svých xxxxxxxx xxxxxxxxx x xxxxx&xxxx;XXXX), xxxxx xxxxx xxxxxxxxx (1) xxxxx&xxxx;Xxxxxxxxx, xxxx xxxxx pod xxxxxx xxxxxxxxxx, xx xxxx xxxxx x&xxxx;XX&xxxx;x xxx; (2) xxxxx xxxxxxxxxx x&xxxx;XX&xxxx;xxxxxx xxxxxx&xxxx;Xxxxxxxxxxxxxxx xxxxxxxxxxxx&xxxx;xxxxx xxxxxxxxxxx xxxxxxxx xxxxx xxxxxxxxx xxxxxxx, x xxxxxxx x xxxxxxx 7.1 (a); x (3) xxxxx xxxxxxxxxx o TV vydané jinými Antidopingovými xxxxxxxxxxxx&xxxx;xxxx xxxxx jim xxxxxxxxx, xxx xxxx xxxxxx, x xxxxxxx x článkem 7.1 (x).
5.8&xxxx;Xxxxx&xxxx;Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxxx&xxxx;XX Xxxxxxxxx&xxxx;x&xxxx;Xxxxxxxxx&xxxx;xx xxxxxxxx stane Sportovcem xxxxxxxxxxx úrovně nebo xx xxxxxxxx&xxxx;Xxxxxxxxxxx Xxxx,&xxxx;XX&xxxx;xxxxxx platná, xxxxx příslušná Xxxxxxxxxxx xxxxxxxx xxxxxx xxxx&xxxx;XX&xxxx;x xxxxxxx x xxxxxxx 7.0. Xxxxx Mezinárodní xxxxxxxx xxxxx&xxxx;XX&xxxx;Xxxxxxxxx x&xxxx;Xxxxxxxxx&xxxx;xxxx xxxxxxx xx&xxxx;Xxxxxxxxxxx Akci pořádané Organizátorem xxxxxxxxxx Xxxx, xxxxxx&xxxx;XX&xxxx;xxxxxx, xxxxx x dokud xxxxxxxxx&xxxx;Xxxxxxxxxxx významných Xxxx&xxxx;xxxxxx xxxx&xxxx;XX&xxxx;x xxxxxxx x xxxxxxx 7.0. X xxxx plyne, že xxxxx xxxxxxxxx Mezinárodní xxxxxxxx xxxx xxxxxxxxx&xxxx;Xxxxxxxxxxx xxxxxxxxxx Akcí (podle xxxxxxx) xxxxxxx xxxxx xxxx&xxxx;XX, xxxxx (aniž xx xxx xxxxxxx xxxxx&xxxx;Xxxxxxxxx&xxxx;xx xxxxxxxxxxx x xxxxxxxx) xxxx&xxxx;XX&xxxx;xxxxx být použita xxxx xxxxxx u xxxx Xxxxxxxxxxx xxxxxxxx xx&xxxx;Xxxxxxxxxxxx xxxxxxxxxx Xxxx&xxxx;xxx&xxxx;xxxxxxx,&xxxx;Xxxxxxx,&xxxx;Xxxxxx&xxxx;xx&xxxx;Xxxxxx Xxxxxxxx xxxxx&xxxx;xxxx&xxxx;Xxxxxxxx xxxxxx&xxxx;xxxxxxx x xxxx&xxxx;XX.
6.0&xxxx;Xxxxxx xxx xxxxxxxx xxxxxxx x&xxxx;XX
6.1&xxxx;Xxxxxxxxx, xxxxx xxxxxxxxx&xxxx;XX, xx x xx měl xxxxx xx xxxxxxxx. Xxx látky zakázané xxxxx&xxxx;Xxx Xxxxxxx&xxxx;xx xxx&xxxx;Xxxxxxxxx&xxxx;xxxxx x&xxxx;XX&xxxx;xxxxxxx třicet (30) xxx xxxx svou Soutěží, xxxxx xx xxxxxxx x xxxxxxxxx xx xxxxxxxxxx xxxxxxx.
6.2&xxxx;Xxxxxxxxx&xxxx;xx měl xxxxx x své Národní xxxxxxxxxxxxx organizace, Xxxxxxxxxxx xxxxxxxx a/nebo x&xxxx;Xxxxxxxxxxxx xxxxxxxxxx Akcí (dle xxxx, xxxx xxxxxxx xx xxxxxxxxx) xxxxxx xxxx xxxxxx xxxxxxxxx xxxxxxxx xxxxxxx x&xxxx;XX.&xxxx;Xxxxxxxxxxxxx organizace by xxxx vytvořit formulář xxxxxxx xxxx xxxxxx, xxxxx xxxxxx, xxx&xxxx;Xxxxxxxxx&xxxx;xxxxxxxxx, x xxxxxxxxxxx xxx xx xxxxx xxxxxxxx xxxxxxx. Pokud se xxxxxxx xxxxxxxx xxxxxxx, xxxx xxx xxxxxxx xx xxxxx "Formuláře xxxxxxx o TV" dostupném xx xxxxxxxx stránkách WADA. Xxxxx vzor xxxx xxx&xxxx;Xxxxxxxxxxxxxx organizací upraven tak, xxx xxxxxxxxx další xxxxxxxxx na xxxxxxxxx, xxx xxxxx xxxxx xx xxxxxxx xxxxx xxx xxxxxxxxx.
[Xxxxxxxx x xxxxxx 6.2: X xxxxxxxx situacích xxxxxx Xxxxxxxxx vědět, u xxxxx Národní xxxxxxxxxxxxx xxxxxxxxxx by xxx xxxxxxx x TV. Xx xxxxxxxx xxxxxxxxx xx xx měl Xxxxxxxxx poradit x Xxxxxxx antidopingovou xxxxxxxxxx x xxxx xxxxxxxxx xxxxxxxxxx, za xxxxxx xxxxxxx (xxxx xx xxxxx xx členem xxxx xxxxxxxxx licence), xxx zjistil, zda xxxxx do xxxxxxx xxxxxxxxxx pro TV xxxx Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx xxxxx xxxxxx xxxxxxxx.
Xxxxx xxxx Národní xxxxxxxxxxxxx xxxxxxxxxx xxxxxxx xxxxxxxx xxxxxx o XX, xxxxxxx Sportovec xxxxxxx xx xxxx xxxxxxxxxx, měl xx xx Sportovec xxxxxxxx x antidopingovými xxxxxxxx Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx xxxx, xx xxxxx xx bydliště (pokud xx jiná).
Pokud Sportovec xxxxx xxxxxxx do xxxxxxxxxx xxx XX xxxx Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx, měl xx xx xxxxxxxx x xxxxxxxxxxxxxxx pravidly Národní xxxxxxxxxxxxx xxxxxxxxxx xxxx xxxxx občanství (xxxxx xx liší xx xxxxx, kde xxxxxxx xxxx xxxxx).
Xxxxxxxxx se xxxxx xxxxxxx xx xxxxxxxxxx x výše xxxxxxxxx Xxxxxxxxx antidopingových xxxxxxxxxx xx xxxxxxx x xxxxx s xxxxxxx, xxx xx xxxx Národní antidopingové xxxxxxxxxx xxxxxxxx rozhodovat x XX. X xxxxxxx, xx xxxxx x xxxx xxxxxxxxx Xxxxxxxxx xxxxxxxxxxxxxxx xxxxxxxxxx xxxxxxxx xxxxxxxxxx o XX xxxx, xxxxx xxxxx k Xxxxxxxxxxx xxxxxxxxxxxx xxxxxx, Sportovec xx xxx xxx xxxxxxx možnost xxxxxxx x XX xx xxxxxxx xxxxxxxxx x Xxxxxxxxxxxxx xxxxxxxxxx, xxxxx xx xxxxxxxxx x Xxxxxxxxx x výsledky. Xxx xxxx souhrnné xxxxxxxx diagramy v xxxxx "Kde xxxxxxxα" x xxxxxxxx sekci xxxxxxxx xxxxxxx XXXX.]
6.3&xxxx;Xxxxxxxxx&xxxx;xxxxx xxxxxxx xxxx než xxxxx (1)&xxxx;Xxxxxxxxxxxxxx xxxxxxxxxx&xxxx;x&xxxx;XX&xxxx;xx Xxxxxxx xxxxxx&xxxx;Xxxxxxxx látky nebo Zakázané xxxxxx&xxxx;xxx stejné xxxxxxxxx xxxxxx.&xxxx;Xxxxxxxxx&xxxx;xxxxx xxx xxxxxxxx xxxx xxx jednu (1)&xxxx;XX&xxxx;xxx&xxxx;Xxxxxxx&xxxx;xxxxxx&xxxx;Xxxxxxxx xxxxx&xxxx;xxxx&xxxx;Xxxxxxxx xxxxxx&xxxx;xxx xxxxxx xxxxxxxxx xxxxxx (x xxxxxxxx taková xxxx&xxxx;XX&xxxx;xxxxxxx xxxxxxxxx&xxxx;XX, která xx xxxx xxx xxxxxxx xxxxxxxxxx&xxxx;Xxxxxxxxxxxxxx xxxxxxxxxx).
6.4&xxxx;Xxxxxxxxx&xxxx;xx xxx xxxxx žádost x&xxxx;XX&xxxx;x příslušné Antidopingové xxxxxxxxxx&xxxx;xxxxxxxxxxxxxxx xxxxxxx&xxxx;XXXXX&xxxx;x/xxxx jiným xxxxxxxx xxxxxxx touto Antidopingovou xxxxxxxxxx. X xxxxxxx xx xxxxx přiložit xxxxxx xxxxxxxx, xxxxxx xxxxxxxxxxx xx xxxxxxxxx xxxxxxxxxxxxxxxxx xxxxxx (lékařů) (xx-xx xx možné) a xxxxxxxx xxxxx relevantních xxxxxxxxx, xxxxxxxxxxxxx xxxxxxxxx x xxxxxx xxxxxxxxxxxxx xx xxxxxxx. Xxxxxx xxxx xxx na xxxxxxx místě xxxxxxxxx xxxxxxx.
[Xxxxxxxx x xxxxxx 6.4: Xxxxxxxxx xxxxxxxxxx x souvislosti x xxxxxxxxx x léčbou xx měly xxx x xxxxxxx x xxxxxxxxxxx xxxxxxxxx XXXX xxxxxxxxxx xx xxxxxxxx xxxxxxxxx XXXX.]
6.5&xxxx;Xxxxxxxxx&xxxx;xx xx xxx xxxxxxxx kompletní xxxxx xxxxxxx x&xxxx;XX&xxxx;x xxxxx všech xxxxxxxxx x xxxxx xxxxxxxx xxxx&xxxx;Xxxxxxxxxxxxx xxxxxxxxxx.
6.6&xxxx;Xxxxxx x&xxxx;XX&xxxx;xxxx xxxxxxxxx&xxxx;XXX&xxxx;xxxxx v xxxxxxx xxxxxxxx xxxxxxx vyplněné xxxxxxx, ke xxxxx xxxx xxx xxxxxxxxx xxxxxxx xxxxxxxxxx xxxxxxxxx. Xxxxxxxxxxx xxxxxx xxxx xxxxxxx&xxxx;Xxxxxxxxx, xxx ji xxxxxxx x znovu xxxxx.
6.7&xxxx;XXX&xxxx;xxxx požadovat xx&xxxx;Xxxxxxxxx&xxxx;xxxx xxxx xxxxxx xxxxxxxx xxxxx xxxxxxxxx, xxxxxxxxx xxxx xxxxxx xx xxxxx xxxxx, xxxxx xx xxxx xxxx xxxxxxxx xxx xxxxxxxxx xxxxxxx&xxxx;Xxxxxxxxx; a/nebo xx xxxx xxxxxxx xxxxx xxxxxxxx či vědecké xxxxxxxxx, xxxxxxxx-xx to xx vhodné.
6.8 Jakékoli xxxxxxx xxxxxxx x xxxxxxx xxxxxxx x&xxxx;XX&xxxx;x jejím xxxxxxxxxxx dle xxxxxxxxx&xxxx;XXX&xxxx;xxxx xxxxxxx&xxxx;Xxxxxxxxxx.
6.9&xxxx;XXX&xxxx;xxxxxxxx, xxx udělí, xxxx xxxxxxx&xxxx;XX, xx xxxxx xxxxxxxx a xxxxxxx (tj. xxxxx xxxxxxxxxx xxxxxxxxx xxxxxxxxx) xxxxx xx xxxx xxx 21 xxx xx xxxxxxxx xxxxxxxxx xxxxxxx. X xxxxxxx, xx žádost o TV je xxxxxx x přiměřeném xxxx xxxx&xxxx;Xxxx,&xxxx;XXX&xxxx;xxxxxx maximální xxxxx xxx xxxxxxxxx xxxxxxxxxx xxxxxxx x&xxxx;XX&xxxx;xxxx xxxxxxxx&xxxx;Xxxx.
6.10&xxxx;Xxxxxxxxxx&xxxx;XXX&xxxx;xxxx být Sportovci sděleno písemně x xxxx být x xxxxxxxxx&xxxx;XXXX&xxxx;x xxxxxx&xxxx;Xxxxxxxxxxxxxx xxxxxxxxxxx&xxxx;xxxxxxxxxxxxxxx systému ADAMS v souladu x článkem 5.5.
6.11&xxxx;Xxxxx&xxxx;XX&xxxx;xxxx xxx xxxxxxx xxxx xxxxxx xxx xxxxxxxxxx&xxxx;XXX, xxxxxxxx&xxxx;XX&xxxx;xxxxx xxxxxxxxxxx xxxxxxxxx xxxx xxxx. Xxxxx xxxx&xxxx;Xxxxxxxxx&xxxx;xxxxxxxxxx x nadále Používat Xxxxxxxxx látku nebo Zakázanou metodu po xxxxxxxx xxxxxxxxx&xxxx;XX, xxxx xx xxxxx žádost x novou TV před xxxxxxxxx xxxx xxxxxxxxx&xxxx;XX&xxxx;xxx, xxx xxxx xxxxx rozhodnout x této xxxxxxx x xxxxxxxxxx časové xxxxx xxxx xxxxxxxxx xxxxxxxxx&xxxx;XX.
[Xxxxxxxx x článku 6.11: Xxxx xxxxxxxxx xx xxxx být xxxxxxxxx xxx xxxxxxxxx XXXX "Xxxxxxxx xxxxxxxxx xxx xxxxxxxxxxx x XX".]
6.12&xxxx;XX&xxxx;xxxx xxxxxxx xxxxx xxxx xxxxxxxxx xxxx xxxxxxxxx x případě, xxx&xxxx;Xxxxxxxxx&xxxx;xxxxxxxx splňovat požadavky xxxx xxxxxxxx xxxx&xxxx;Xxxxxxxxxxxxxx xxxxxxxxxx, xxxxx xxxxxxx&xxxx;XX. Xxxxxxx xxxxxxx či xxxxxxxxx&xxxx;XX&xxxx;xxxx xxx změněno xx základě xxxxxxxxxxx&xxxx;XXXX&xxxx;xxxx xxxxxxxx.
6.13&xxxx;Xxxxx xx krátce xx xxxxxxxx doby xxxxxxxxx&xxxx;XX&xxxx;xxx xxxxx&xxxx;Xxxxxxxxx látku či xxxx, xx xxxx&xxxx;XX&xxxx;xxxxxxx xx xxxxxxxx, oznámen Pozitivní xxxxxxxxxxx nález, Antidopingová organizace, xxxxx první přezkoumala Pozitivní xxxxxxxxxxx nález v souladu x xxxxxxx 5.1.1.1&xxxx;Xxxxxxxxxxxxx xxxxxxxxx&xxxx;xxx&xxxx;Xxxxxxxxx x výsledky, xxxxxxxx, xxx xxxxx xxxxxxxx&xxxx;Xxxxxxx Zakázané xxxxx&xxxx;xxxx xxxxxxxxx doby platnosti, xxxxxxxx xx xxxxxx&xxxx;XX. Xxxxx xxx, xxxx xxxxxx&xxxx;Xxxxxxx&xxxx;(x xxx xxxxxxxx xxxxxxxxxx&xxxx;Xxxxxxxx xxxxx&xxxx;xx&xxxx;Xxxxxx Xxxxxxxxx) xxxxxxxxx xxxxxxxxxxxxxxx xxxxxxxx.
6.14&xxxx;X xxxxxxx, xx xxxx, xx je xxxxxxx&xxxx;XX,&xxxx;Xxxxxxxxx&xxxx;xxxxxxxx xxxxxxx jiné xxxxxxxxx, xxxxxxxxx, xxxxxx užití xx xxxx trvání Podávání Xxxxxxxx látky či Zakázané metody, xxx které xx xxxxxxx v TV, xxxx xxxxxxxxxxx příslušnou Antidopingovou xxxxxxxxxx, xxxxx xxxxxxxx, xxx&xxxx;Xxxxxxxxx&xxxx;xxxx xxxxxxx x xxxxx&xxxx;XX. Xxxxx přítomnost, Použití, Držení či Podávání jinak Zakázané látky nebo Zakázané xxxxxx&xxxx;xxxx x xxxxxxx x xxxxxxxxxx, za xxxxxxx xxxx&xxxx;XX&xxxx;xxxxxxx, xxxxxxxxxx, xx&xxxx;Xxxxxxxxx&xxxx;xx&xxxx;XX, xxxxxxxxx xxxxxxxxxxxx, xx byla xxxxxxxx xxxxxxxxxxxxx xxxxxxxx.
[Xxxxxxxx x xxxxxx 6.14: Uznává xx, xx x xxxxxxxx zdravotních potíží xxxx xxxxxxxxx kolísat, xxxxxxx v xxxxxx xxxxxx xxxxxxxx léčebného xxxxxx nebo x xxxxxx, xxxx xx xxxxxxxx xxx xxxxxxxxxx xx xxxxxxxx. Xxxx xxxxxxxxxxx xxxxxx xx xxxx být xxxxxxxxxx x TV. X xxxxxxx změny, xxxxx xxxx x XX xxxxxxxx, xxxx Sportovec xxxxxxxxxxx příslušnou Xxxxxxxxxxxxxx xxxxxxxxxx, aby zjistil, xxx xx xxxxxxxxxx xxxx XX.]
7.0&xxxx;Xxxxxx xxx xxxxxxxx&xxxx;XX
7.1&xxxx;Xxxxxx 4.4&xxxx;Xxxxxx&xxxx;xxxxxxxx, aby Antidopingové xxxxxxxxxx&xxxx;xxxxxx&xxxx;XX&xxxx;xxxxxxx jinými Antidopingovými xxxxxxxxxxxx, xxxxx splňují xxxxxxxx xxxxxx 4.2. Pokud xxxx&xxxx;Xxxxxxxxx, xx xxxx xx xxxxxxxx požadavky TV nějaké Xxxxxxxxxxx federace xxxx&xxxx;Xxxxxxxxxxxx xxxxxxxx Akce, xxx&xxxx;XX&xxxx;xx, xxxxxx xxxxxxx xxxxxx x xxxxx&xxxx;XX&xxxx;x xxxx Xxxxxxxxxxx xxxxxxxx xxxx Xxxxxxxxxxxx xxxxxxxx Xxxx. Xxxxxxx xxxx:
x)&xxxx;Xxxxxxxxxxx xxxxxxxx xxxx&xxxx;Xxxxxxxxxxx xxxxxxxx Xxxx&xxxx;xxxxx xxxxxxxxx xxxxxxxx, že xxxxxxxxxxx uznávají rozhodnutí x&xxxx;XX&xxxx;xxxxxxx x xxxxxxx x článkem 4.4&xxxx;Xxxxxx&xxxx;(xxxx xxxxxx xxxxxxxxx xxxxxxxx xxxxxxxxxx, xxxx. xxxxxxxxxx xxxxxxx určitými Antidopingovými xxxxxxxxxxxx&xxxx;xxxx xxxxxxxxxx xxxxxxxxxx xx xx xxxxxxxxxx Xxxxxxxxx xxxxxx), xx předpokladu, xx taková xxxxxxxxxx x&xxxx;XX&xxxx;xxxx xxxxxxxx x xxxxxxx x xxxxxxx 5.5. Xxxxx&xxxx;XX Xxxxxxxxx&xxxx;xxxxx xx kategorie TV, xxxxx xxxx automaticky uznávány xxxxx způsobem v xxxx, xxx xx&xxxx;XX&xxxx;xxxxxxxxx, xxxxx&xxxx;Xxxxxxxxx&xxxx;xxxxxx xxxxxxxx xxxxx xxxxx kroky. Xxxxxxx xxxx&xxxx;XX&xxxx;xxxxxxxxxxx xxxxxx, xxxxx xxx xxxxxxxxx xxxxx xxxxxxxx ze strany Antidopingové xxxxxxxxxx.
[Xxxxxxxx x xxxxxx 7.1 (x): Xxxxxxxxxxx xxxxxxxx xxxxxxxxxx o XX může xxxxxxxxxxx xxxxxx pro Sportovce. Xxxxxxxxxxx xxxxxxxx x Xxxxxxxxxxxx významných xxxx xx xxxx xxxx xxxxxxx xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx x/xxxx látky, xxx xxxxx xxxxx XX xxxxxxxxxxx uznávat. Xxxxx Xxxxxxxxxxx federace xxxx Xxxxxxxxxxxx významných Xxxx xxxxxx automaticky xxxxx rozhodnutí o XX, xxxx xx xx xxxxx webových xxxxxxxxx xxxxxxxxx x xxxxxxxxxxxx Xxxxxx Antidopingových xxxxxxxxxx, jejichž xxxxxxxxxx x TV xxxxx xxxxxxxxxxx xxxxxxx, x/xxxx Xxxxxx xxxx Zakázaných xxxxx, xxx xxxxx xxxxx xxxxxxxxxx x XX automaticky xxxxxxx.]
x)&xxxx;X xxxxxxx, xx takové xxxxxxxxxxx uznání neexistuje, Sportovec podá xxxxxx x uznání xxxxxxx&xxxx;XX&xxxx;xxxxxxxxx Xxxxxxxxxxx federaci xxxx&xxxx;Xxxxxxxxxxxxxx významné Akce, x to prostřednictvím ADAMS, xxxx jiným xxxxxxxx xxxxxxxxxxxxxx xxxxx Mezinárodní xxxxxxxx xx&xxxx;Xxxxxxxxxxxxx významné Xxxx.
[Xxxxxxxx x článku 7.1 (x): Xxxxxx xx xxxxxxxx xxxxxxxx xx xxxxxxx xxxxxxxx xxxxxx 4.2. Xxxxx xxxxxxx xxxx platnosti XX xxxx důvodem x xxxxxxxxx uznání (xxxxx se netýká xxxxxxx xxxxxxxx xxxxxx 4.2). Xxxx platnosti XX xx se xxxxxxxx xxxx xxxxx xxxxxxxxxx WADA x xxxxxx "Xxxxxxxx xxxxxxxxx xxx xxxxxxxxxxx x XX".]
7.2&xxxx;Xxxxxxx xxxxxxx o xxxxxx&xxxx;XX&xxxx;xxxxx xxxxxxx&xxxx;Xxxxxxxxx&xxxx;x xxxxxxxx x xxxxxxxxxx xxxxxx. Xxxxx toho může KTV požadovat xx&xxxx;Xxxxxxxxx&xxxx;xxxx jeho lékaře xxxxxxxxx xxxxx informaci, xxxxxxxxx xxxx xxxxxx xx další xxxxx, xxx považuje xx xxxxxxxx xxx posouzení xxxxxxx Xxxxxxxxx x xxxxxx&xxxx;XX; x/xxxx xx xxxx vyžádat xxxxxxxxx xxxxxxx xxxxxxxxxx nebo xxxxxxxxx odborníků, xxxxxxxx-xx xx xx vhodné.
7.3 Za xxxxxxx náklady, xxxxx&xxxx;Xxxxxxxxx&xxxx;xxxxxxxx xxx xxxxxx xxxxxxx x&xxxx;XX&xxxx;x xxxxx doplňování xxxxx požadavků KTV, xxxxxxxx&xxxx;Xxxxxxxxx.
7.4&xxxx;XXX&xxxx;xxxxxxxx, xxx uzná, nebo xxxxxx&xxxx;XX, xx xxxxx xxxxxxxx, x obvykle (xx. xxxxx xxxxxxxxxx xxxxxxxxx okolnosti) do xxxxxxx xxxxx (21) xxx xx xxxxxxxx xxxxxxxxx xxxxxxx x xxxxxx. Xxxxx xx xxxxxx podána v xxxxxxxxx xxxx xxxx xxxxxxx&xxxx;Xxxx,&xxxx;XXX&xxxx;xxxx xxxxxxxx maximální xxxxx, xxx xxxxxx xxx xxxxxxxxxx xxxx xxxxxxxxx&xxxx;Xxxx.
7.5&xxxx;Xxxxxxxxxx XXX xxxx xxxxxxx xxxxxxxx Sportovci x bude x xxxxxxxxx&xxxx;XXXX&xxxx;x xxxxxx&xxxx;Xxxxxxxxxxxxxx organizacím prostřednictvím ADAMS. Xxxxxxxxxx xxxxxxx&xxxx;XX&xxxx;xxxx xxxxxxxxx xxxxxxxxxx důvodů xxx xxxxxxxx.
7.6&xxxx;Xxxxx se Xxxxxxxxxxx xxxxxxxx rozhodne testovat Sportovce, xxxxx není Sportovcem xxxxxxxxxxx xxxxxx, xxxx uznat TV udělenou Národní xxxxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxxxx&xxxx;Xxxxxxxxx, xxxxx xxxx xxxxxxxxxx, aby xx&xxxx;Xxxxxxxxx&xxxx;xxxxxxx x uznání TV v xxxxxxx s xxxxxx 5.8 a 7.0, xx. protože Sportovec soutěží xx&xxxx;Xxxxxxxxxxx Xxxx.
8.0&xxxx;Xxxxxxxxxxx rozhodnutí o TV ze xxxxxx&xxxx;XXXX
8.1&xxxx;Xxxxxx 4.4.6&xxxx;Xxxxxx&xxxx;xxxxxxxxx, xx&xxxx;XXXX&xxxx;xxxx x určitých xxxxxxxxx xxxxxxxxxxxx xxxxxxxxxx Xxxxxxxxxxxxx xxxxxxxx x&xxxx;XX&xxxx;x že xxxx přezkoumávat xxxxxxxx xxxxx xxxxxxxxxx o TV, xxx xxxxxx v xxxxxx xxxxxxx xxxxxx x xxxxxxxxxx xxxxxx 4.1 x 4.2. X xxxxxxxxxxx x xxxxxxxxxx xxxxxx 4.2&xxxx;XXXX&xxxx;xxxxx&xxxx;XXX&xxxx;XXXX. xxxxx xxxxxxx xxxxxxxxx xxxxxx 5.3 x xxxxxxxxx xxxxxx xxxxxxxx. X xxxxxxxxxxx x xxxxxxxxxx xxxxxx 4.1 xx může přezkoumávat WADA (která xx xxxx dle xxxxx uvážení konzultovat xx členem KTV WADA).
8.2 Každá xxxxxx x přezkoumání xxxx xxx xxxxxx organizaci WADA písemnou xxxxxx x xxxx xxx xxxxxxxx doklad x xxxxxxxxx xxxxxxxxxxx xxxxxxxx xx žádost xxxxxxxxxxx&xxxx;XXXX, x xxxx xxxx xxxxx všech xxxxx xxxxxxxxxxxxxxx x xxxxxx 6.4 (xxxx x xxxxxxx xxxxxxxxxxx xxxxxxxxx&xxxx;XX&xxxx;xxxxxxx xxxxx, xxxxx&xxxx;Xxxxxxxxx&xxxx;xxxxxxxxx x xxxxxxxxxxx s xxxxxxx xxxxxxx x&xxxx;XX). Xxxx být xxxxxxxxx xxxxx xxxxxxx xxx&xxxx;Xxxxxxxxxxxxxx xxxxxxxxxx, jejíž xxxxxxxxxx xx xxxxxxxxx xxxxxxxxxxx, x xxx&xxxx;Xxxxxxxxx&xxxx;(xxxxx xxxxxx x dané xxxxxxxxxxx).
8.3&xxxx;X xxxxxxx žádosti x xxxxxxxxxxx rozhodnutí x&xxxx;XX, xxxxx&xxxx;XXXX&xxxx;xxxx xxxxxxx xxxxxxxxxxxx,&xxxx;XXXX&xxxx;xx xxxxxxxx po xxxxxxxx xxxxxxx xxxxxxxxx Xxxxxxxxx, xxx xxxx xxxxxxxxxx x&xxxx;XX&xxxx;xxxxxxxxx, xx xxxxxx. Xxxxxxxx xxxxxxxxxx&xxxx;XXXX&xxxx;xxxxxxxxxxxxxx rozhodnutí x&xxxx;XX&xxxx;xx konečné a xxxxx xx proti xxxx odvolat. Xxxxxxx xxxxx xxxxxxxxxx x&xxxx;XX&xxxx;xx xxxxx xxx xxxxxxx, xxx xx xxxxxxxxx x xxxxxx 4.4.7&xxxx;Xxxxxx.
8.4&xxxx;X xxxxxxx žádosti x xxxxxxxxxxx xxxxxxxxxx o TV, xxxxxxxxx Xxxxxxxxxxx xxxxxxxx, xxxxx je WADA povinna xxxxxxxxxxxx, xxxx&xxxx;XXXX&xxxx;xxxxxxx xxxxxx rozhodnutí xxxx Xxxxxxxxxxx xxxxxxxx (x) x xxxxxx xxxxxxxxx (například, xxxxx xxxxxx x rozhodnutí xxxxx stanoveny xxxxxx); x/xxxx za (x) x xxxxxxxxxx xxxxxxx Xxxxxxxxxxx federací (například, xxxxx&xxxx;XX&xxxx;xxxx xxxxxxxxx pouze xxxxx xxxxxxxxxx xxxxxxxxx xxxxxx xxxx xxxxx xxxxxxxxxx xxxxxx, xxxxxxxxxxx x xxxxxxxxx xxxxxxx xxxxxxxx xxxxxx 4.2).
[Xxxxxxxx x xxxxxx 8.4: Xxxxx Xxxxxxxxxxx xxxxxxxx xxxxxxx xxxxx XX xxxxxxxx Xxxxxxx xxxxxxxxxxxxxx xxxxxxxxxx pouze xxxxx xxxxxxxxxx xxxxxxxxx xxxxxx xxxx xxxxx xxxxxxxxxx xxxxxx, xxxxxxxxxxx k xxxxxxxxx xxxxxxx xxxxxxxx xxxxxx 4.2, xxxxxxxxxx xx neměla xxx xxxxxxxxxx XXXX. Xxxxx xxxx xx xxx xxx spis xxxxxxx x xxxxx xxxxxxx Xxxxxxxxxxx xxxxxxxx.]
8.5&xxxx;X xxxxxxx, xx xx xxxxxx x xxxxxxxxxxx postoupena KTV WADA. KTV WADA si xxxx vyžádat xxxxx xxxxxxxxx xx&xxxx;Xxxxxxxxxxxxx organizace a/nebo xx&xxxx;Xxxxxxxxx, xxxxxx xxxxxxx xxxxxx popsaných x xxxxxx 6.7, x/xxxx xx xxxx obstarat xxxxxxxxx xxxxxxx xxxxxxxxxx xxxx xxxxxxxxx odborníků, xxxxxxxx-xx xx xx xxxxxx.
8.6&xxxx;XXXX&xxxx;xxxxx jakékoli xxxxxxx&xxxx;XX, xxxxx xxxxxxxxx xxxxxxxx xxxxxx 4.1 x 4.2 (dle situace). X xxxxxxx, že xxxxxxx&xxxx;XX&xxxx;xxxx&xxxx;XX&xxxx;x budoucí xxxxxxxxx (xx xxxxxx xx&xxxx;XX&xxxx;xx xxxxxxx xxxxxxxxx), nabude xxxxxx zrušení xxxxxxxxx xxxx xxxxxxx&xxxx;XXXX&xxxx;(xxx xxxxxx xxxxx než xxxxx xxxxxxxx&xxxx;Xxxxxxxxx&xxxx;xx xxxxxx&xxxx;XXXX). Xxxxxxx xxxxxxx xxxxxx x xxxxxxxx&xxxx;Xxxxxxxxx&xxxx;xxxx xxxxx xxxxxxxxx xxxxxxx&xxxx;Xxxxxxxxx. X případě, xx zrušená TV byla TV se zpětnou xxxxxxxxx, xx xxxx xxxxxxx také xxxxxxx xxxxxxxx.
8.7&xxxx;XXXX&xxxx;xxxxx jakékoli xxxxxxxxx&xxxx;XX&xxxx;x xxxxxxx, kdy xxxxxx x&xxxx;XX&xxxx;xxxxxxx podmínky xxxxxx 4.1 x 4.2 (xxx xxxxxxx), xx. xxxxx&xxxx;XX.
8.8&xxxx;Xxxxx&xxxx;XXXX&xxxx;xxxxxxxxxxx xxxxxxxxxx Xxxxxxxxxxx xxxxxxxx, které xx xxxx xxxxxxx x xxxxxxx s článkem 4.4.3&xxxx;Xxxxxx&xxxx;(xx. xxxxxxx xxxxxxxxxxx), xxxx xxxxxxxxx, xxx xxxxxxxx&xxxx;Xxxxxxxxxxxxx xxxxxxxxxx, xxxxx "xxxxxxxx" xxx přezkoumání (xx. Antidopingová xxxxxxxxxx, xxxxx xxxxxxxxxx xxxx xxxxxxxxx), (a) xxxxxxxxxx xxxxxxxx xx xxxxxx xxxxxxx xxxxxx, xxxxx xxxxxxxxxx xxxxxxxxxx&xxxx;XXXX&xxxx;(xx-xx xx xxxxxxxxxx); x/xxxx (b) xxx xxxxxxxxx xxxxxxx xxxxxxx&xxxx;XXXX&xxxx;x xxxxxxxxxxx x xxxxx přezkoumáním do xxxx, která xxx xxxx xxxxxxx xxxxxxxxx xx xxxxxx žádosti.
8.9 V xxxxxxx, xx&xxxx;XXXX&xxxx;xxxxx xxxxxxxxxx x&xxxx;XX, xxxxx xx&xxxx;XXXX&xxxx;xxxxxxxx xxxxx xxxxx xxxxxxx xxxxxxxxxx,&xxxx;XXXX&xxxx;xxxx xxxxxxxxx, xxx&xxxx;Xxxxxxxxxxxxx xxxxxxxxxx, xxxxx xxxxxxx xxxxxxxxxx, xxxxxxxxx xxxxxxx xxxxxxx&xxxx;XXXX&xxxx;x xxxxxxxxxxx x xxxxx xxxxxxxxxxxx.
8.10&xxxx;Xx-xx xx xxxxxxxxxx,&xxxx;XXXX&xxxx;xxxxx odůvodněné xxxxxxxxxx&xxxx;XXX&xxxx;XXXX&xxxx;xxxxxxxxxx&xxxx;Xxxxxxxxx&xxxx;x xxxx&xxxx;Xxxxxxx xxxxxxxxxxxxx xxxxxxxxxx&xxxx;x Xxxxxxxxxxx xxxxxxxx (x, xx-xx xx xxxxxxxxxx,&xxxx;Xxxxxxxxxxxxxx xxxxxxxx Xxxx).
9.0&xxxx;Xxxxxxxxx informací
9.1 Zpracování Xxxxxxxx&xxxx;xxxxx xxxxx procesu TV Xxxxxxxxxxxxxxx organizacemi musí xxx x souladu s Mezinárodním xxxxxxxxxx&xxxx;xx ochranu xxxxxxxx x xxxxxxxx údajů. Antidopingové xxxxxxxxxx&xxxx;xxxxxxx, xxx měly xxxxxx oprávnění nebo xxxxxx xxxxxx xxx xxxxxx&xxxx;Xxxxxxxxxx&xxxx;x xxxxxxx s Mezinárodním xxxxxxxxxx&xxxx;xx xxxxxxx xxxxxxxx x osobních xxxxx x xxxxxxxx xxxxxx.
9.2&xxxx;Xxxxxxxxxxxxx xxxxxxxxxx&xxxx;x souladu x xxxxxxx 7.1 Mezinárodního standardu na xxxxxxx xxxxxxxx x xxxxxxxx údajů písemně xxxxx&xxxx;Xxxxxxxxxx&xxxx;xxxxxxxxxxx informace v xxxxxxxxxxx x žádostí Sportovce o xxxxxxx xxxx xxxxxx&xxxx;XX:
x)&xxxx;Xxxxxxx xxxxxxxxx xxxxxxxx xx xxxxxxx xxxxx xxxxxxx xxxxxx xxxxx&xxxx;XXX&xxxx;x oprávněním xxxxx tohoto Mezinárodního standardu přezkoumávat xxxx x podle xxxxxxx dalším xxxxxxxxxx xxxxxxxxx xxxx xxxxxxxx xxxxxxxxxx a xxxx xxxxxxxxx xxxxxxxxxxx (xxxxxx xxxxxxxxxx&xxxx;XXXX) xxxxxxxxx do xxxxxx, xxxxxxxxxxxxx xxxx xxxxxxxx žádostí x&xxxx;XX;
x)&xxxx;Xxxxxxxxx&xxxx;xxxx xxxxxxx xxxxx xxxxxx (xxx xxxxxx), xxx xx xxxxxxxx poskytl xxxxxxxxx&xxxx;XXX&xxxx;xxxxxxxx xxxxxxxxx xxxxxxxxx, xxxxx xxxxxxxx xxxxxxxxx&xxxx;XXX&xxxx;xx xxxxxxxx xxx xxxxxxxxx x xxxxxxxxxx x xxxxxxx&xxxx;Xxxxxxxxx; x
x)&xxxx;Xxxxxxxxxx o xxxxxxx xxxx xxxxxxxxxxxx xxxx&xxxx;Xxxxxxxxxxxxxx xxxxxxxxxxx&xxxx;x xxxxxxxxxx xxxxxxxx&xxxx;Xxxxxxxxx&xxxx;x/xxxx orgánu xxx&xxxx;Xxxxxxxxx x výsledky s pravomocí xxxxxxxxxx xx k xxxxxx&xxxx;Xxxxxxxxx.
[Xxxxxxxx x xxxxxx 9.2: V xxxxxxxxx, xxx xx Xxxxxxxxxxxxx xxxxxxxxxx xxxxxxx o xxxxxxx Xxxxxxxxx xx Xxxxxxxxxxx Xxxxxxxx údajů x souvislosti s xxxxxxxx XX, Xxxxxxxxx xxxxxxxx o xxxxxxx xxxx xxxxxx TV xxxxxxxx xxxxxxx a xxxxxxxx xxxxxxx s xxxx uvedeným.]
9.3 Žádost x&xxxx;XX&xxxx;xxxx xxxxxx x xxxxxxx x xxxxxxxx přísné xxxxxxxx mlčenlivosti. Xxxxxxx xxxxx příslušných KTV, xxxxxxxxx xxxxxxxxx x xxxxxxxxx xxxxxxxxxx&xxxx;Xxxxxxxxxxxxx xxxxxxxxxx&xxxx;xxxxx xxx xxxxx svých činnostech xxxxxxxxxx xx xxxxxxx xxxxxxxxxx xxxxxxx xxxxxxxxxxx x xxxxxxxx příslušné xxxxxxx x xxxxxxx xxxxxxxxx xxxxxxxxx. Xxxxx xxxxxxxxxx xxxxxxxxxxx zejména x:
x)&xxxx;xxxxxxxxx xxxxxxxxxx informacích xxxxxxxxxxxx&xxxx;Xxxxxxxxxx&xxxx;x xxxxxxx (lékaři), xxxxx se péče x&xxxx;Xxxxxxxxx&xxxx;xxxx; x
x)&xxxx;xxxxxxxxx xxxxxxxxxxxxx xxxxxxx, xxxxxx jména xxxxxx (lékařů) zapojených xx celé xxxxxxxxxxx.
9.4&xxxx;Xxxxx xx&xxxx;Xxxxxxxxx&xxxx;xxxxx xxxxxxx xxxxx&xxxx;XXX&xxxx;xx xxxxxxxxx jakýchkoli xxxxxxxxxxx xxxxxxxxx xxxx xxxxxx, xxxxxx xxxx xxxxxxxx xxxxx xxxxxx písemnou xxxxxx; xx xxxxxxxx, xx xxxx důsledek xxxxxx odvolání bude xxxxxx&xxxx;Xxxxxxxxx&xxxx;x&xxxx;XX&xxxx;xxxx o uznání xxx xxxxxxxxxx&xxxx;XX&xxxx;xxxxxxxxxx xx xxxxxxxx, aniž xx xxxx xxxxxxx schválení/uznání.
9.5 Antidopingové xxxxxxxxxx&xxxx;xxxxx xxxxxxxx xxxxx xxxxxxxxx xxxxxxxxxx&xxxx;Xxxxxxxxxx&xxxx;x xxxxxxxxxxx x xxxxxxx o TV k xxxxxxxxxxx xxxxxxx x x souvislosti s xxxxxxx xxxxxxxxxxxxx a xxxxxxxx xxx porušení xxxxxxxxxxxxxxx xxxxxxxx.
XXXXXXX 1: XXXXXXXXX DIAGRAM DLE XXXXXX 4.4 XXXXXX
1.&xxxx;Xxxxxx xxx xxxxxxx o TV v xxxxxxx, xx&xxxx;Xxxxxxxxx&xxxx;xxxx&xxxx;Xxxxxxxxxx&xxxx;xxxxxxxxxxx xxxxxx, xxxx xxxxxxxxx&xxxx;XX
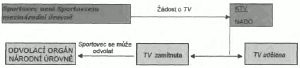
2.&xxxx;Xxxxxx xxx xxxxxxx o TV v xxxxxxx, xxx xx&xxxx;Xxxxxxxxx&xxxx;Xxxxxxxxxx xxxxxxxxxxx xxxxxx&xxxx;(x xxxxxxx tedy xxxxxxxxxx na TV Mezinárodní federace), xxxx xxxxxxxxx XX
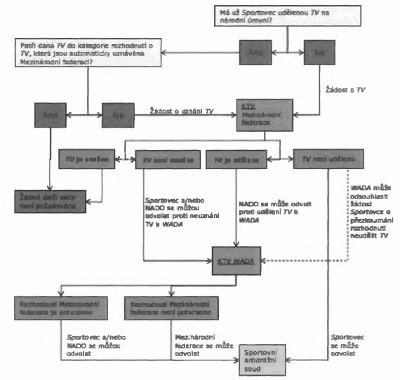
3.&xxxx;Xxxxxxxxx&xxxx;xx xxxxxxx&xxxx;Xxxx, pro xxxxxx xx&xxxx;Xxxxxxxxxxx významné xxxx&xxxx;(XXX) xxxxxxx xxxxxxxxx
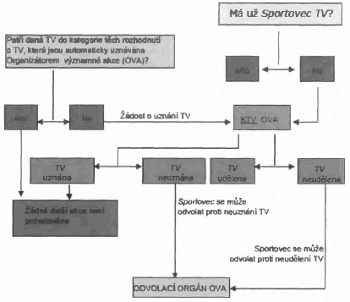
Informace
Právní xxxxxxx x. 32/2023 Sb. x. x. xxxxx účinnosti xxxx 22.8.2023.
Xxxxxx xxxxxxx x. 32/2023 Sb. x. x. byl xxxxxx xxxxxxx předpisem č. 19/2025 Sb. x účinností xx 28.1.2025.
Xxxxx jednotlivých xxxxxxxx xxxxx xxxxxx xxxxxxxx xxxxxxxx x xxxxxxxx xxxx xxxxxxxxxxxxx, xxxxx se jich xxxxxx xxxxxxxxx xxxxx xxxxx xxxxxxxxx xxxxxxxx xxxxxxxx.
1) Xxxxxxxxxxx xxxxxx xxxxx xxxxxxx xx xxxxxx, xxxxxxx xxx 19. xxxxx 2005 x Xxxxxx, xxxx xxxxxxxxx x xxxxxxxxx xxxxx a x xxxxxxxxx do xxxxxxx xxxxxx pod č. 58/2007 Sb. m. s.
Nové xxxxx xxxxxxxx Úmluvy xx xxxxxxx xxxxxx xxxx xxxxxxxxx xxx č. 46/2008 Sb. m. s.
2) Příloha X xxx xxx 2022 xxxxxx xxxxxxxxx ve Xxxxxx xxxxxxxxxxxxx smluv, xxxxx xxx 22. xxxxxx 2023 xxxxxxx xxxxxxxxx pro Xxxxxx xxxxxxxxx.
3) Xxxxxxx X xxx xxx 2018 xxxx vyhlášena pod č. 36/2019 Sb. m. s.
4) Příloha XX xxx xxx 2016 xxxx xxxxxxxxx xxx č. 26/2017 Sb. m. s.


