XXXXXXXXX XXXXXXXX KOMISE (XX) 2021/608
ze dne 14.&xxxx;xxxxx 2021,
kterým se xxxx xxxxxxxxx nařízení (XX) 2019/1793 x&xxxx;xxxxxxxx xxxxxxxxxxxxx xxxxxxxx xxxxxxx x&xxxx;xxxxxxxxxxx xxxxxxxx upravujících xxxxx xxxxxxxx xxxxx x&xxxx;xxxxxxxx xxxxxxx xxxx xx Unie, xxxxxx xx xxxxxxxxx nařízení Xxxxxxxxxx xxxxxxxxxx x&xxxx;Xxxx (XX) 2017/625 x&xxxx;(XX) x.&xxxx;178/2002
(Xxxx x&xxxx;xxxxxxxx pro XXX)
XXXXXXXX XXXXXX,
x&xxxx;xxxxxxx xx Xxxxxxx x&xxxx;xxxxxxxxx Evropské xxxx,
x&xxxx;xxxxxxx xx nařízení Xxxxxxxxxx xxxxxxxxxx x&xxxx;Xxxx (XX) x.&xxxx;178/2002 xx xxx 28.&xxxx;xxxxx&xxxx;2002, xxxxxx xx xxxxxxx obecné xxxxxx a požadavky xxxxxxxxxxxxx xxxxx, xxxxxxx xx Xxxxxxxx xxxx xxx xxxxxxxxxx potravin x&xxxx;xxxxxxx xxxxxxx týkající xx xxxxxxxxxxx xxxxxxxx&xxxx;(1), a zejména xx xx.&xxxx;53 xxxx.&xxxx;1 xxxx. b) xxxxxxxxx xxxxxxxx,
x&xxxx;xxxxxxx xx nařízení Xxxxxxxxxx xxxxxxxxxx x&xxxx;Xxxx (XX) 2017/625 ze xxx 15. března 2017 x&xxxx;xxxxxxxx xxxxxxxxxx x&xxxx;xxxxxx úředních xxxxxxxxxx prováděných s cílem xxxxxxxx uplatňování xxxxxxxxxxxxx x&xxxx;xxxxxxxxxx práva x&xxxx;xxxxxxxx xxxxxxxxxx se xxxxxx xxxxxx x&xxxx;xxxxxxx xxxxxxxxx xxxxxxxx zvířat, zdraví xxxxxxx a přípravků xx xxxxxxx rostlin, o změně xxxxxxxx Evropského xxxxxxxxxx x&xxxx;Xxxx (XX) č. 999/2001, (XX) x.&xxxx;396/2005, (ES) x.&xxxx;1069/2009, (ES) č. 1107/2009, (XX) č. 1151/2012, (EU) x.&xxxx;652/2014, (XX) 2016/429 x&xxxx;(XX) 2016/2031, xxxxxxxx Xxxx (XX) x.&xxxx;1/2005 x&xxxx;(XX) č. 1099/2009 x&xxxx;xxxxxxx Xxxx 98/58/ES, 1999/74/XX, 2007/43/XX, 2008/119/XX x&xxxx;2008/120/XX x&xxxx;x&xxxx;xxxxxxx xxxxxxxx Evropského xxxxxxxxxx x&xxxx;Xxxx (ES) x.&xxxx;854/2004 a (ES) č. 882/2004, xxxxxxx Xxxx 89/608/EHS, 89/662/XXX, 90/425/XXX, 91/496/XXX, 96/23/XX, 96/93/XX a 97/78/ES x&xxxx;xxxxxxxxxx Rady 92/438/XXX (xxxxxxxx x&xxxx;xxxxxxxx xxxxxxxxxx)&xxxx;(2), x&xxxx;xxxxxxx xx čl. 47 xxxx.&xxxx;2 první xxxxxxxxxxx xxxx. x), xx.&xxxx;54 xxxx.&xxxx;4 xxxxx xxxxxxxxxxx xxxx. x) x&xxxx;x) x&xxxx;xx.&xxxx;90 xxxxx pododstavec xxxx. a), b) x&xxxx;x).
xxxxxxxx k těmto xxxxxxx:
|
(1) |
Xxxxxxxxx xxxxxxxx Xxxxxx (EU) 2019/1793&xxxx;(3) xxxxxxx xxxxxxxx xxxxxxxx xx xxxxxxxxx xxxxxxxxxxxxx xxxxxxxx xxxxxxx xxx xxxxxx xxxxxxxx xxxxxxxx x&xxxx;xxxxx xxxxxx xxx xxxxxxxxxxx původu x&xxxx;xxxxxxxx třetích xxxx xxxxxxxxx xx seznamu x&xxxx;xxxxxxx I uvedeného xxxxxxxxxxx xxxxxxxx do Xxxx x&xxxx;xxxxxxxx podmínky xxx xxxxx xxxxxxxx potravin x&xxxx;xxxxx x&xxxx;xxxxxxxx xxxxxxx xxxx xxxxxxxxx xx xxxxxxx v příloze II xxxxxxxxx prováděcího nařízení xx Xxxx x&xxxx;xxxxxx xxxxxx kontaminace xxxxxxxxxx, xxxxxx xxxxxxxxxx, rezidui xxxxxxxxx, xxxxxxxxxxxxxxxxx x&xxxx;xxxxxxx x&xxxx;xxxxxxxxxxxxxxx xxxxxxxxxxx. |
|
(2) |
Xxxxxxxxx xxxxxxxx (XX) 2019/1793 xxxxxxx xxxxxxxxx, xxxxx xxx x&xxxx;xxxxxxx xxxxxx osvědčení xxx vstup xxxxxxx xxxxxxxx x&xxxx;xxxxx xxxxxxxxx x&xxxx;xxxxxxx XX xxxxxxxxx xxxxxxxxxxx xxxxxxxx xx Xxxx, x&xxxx;xxxxxxxx pro xxxxxxxx xxxxxxxx osvědčení x&xxxx;xxxxxxx x&xxxx;xxxxxxxxxxxx xxxxxx. X&xxxx;xxxxxxx x&xxxx;xxxxxxxxxx nařízením Xxxxxx (XX) 2019/1715 (4) xx xxxxxx XXXXXX xxxxxxx xxxxxxx xxx xxxxxx xxxxxxxxx x&xxxx;xxxxxxxx xxxxxxxxxx (XXXXX), xxxxx xxxxxxxx, xxx celý xxxxxx tvorby xxxxxxxxx xxxxxxxx xxxxxxxxxxxx, a tím xx xxxxxxxxx xxxxxx xxxxxxxxx xxxx xxxxxxxx xxxxxxxxx v souvislosti x&xxxx;xxxxxxxx xxxxxxxxxxx. Xxxxxxxxx xxxxxxxx (XX) 2019/1793 proto xxxxxxx xxxxxxx úřední xxxxxxxxx, xxx xx x&xxxx;xxxxxxx s TRACES. |
|
(3) |
Požadavky xx xxxxxxxxxxx stanovené x&xxxx;xxxxxxxxxx xxxxxxxx (EU) 2019/1793 xxxx x&xxxx;xxxxxxx x&xxxx;xxxxxxxxx xxxxxxxxxxx x&xxxx;xxxxxxxxxx xxxxxxxx Xxxxxx (XX) 2019/628&xxxx;(5) xxx xxxxxx osvědčení xxx xxxxx xx Xxxx. Xxxxxxxxx nařízení Xxxxxx (XX) 2020/2235&xxxx;(6) xxxxxxx a nahrazuje prováděcí xxxxxxxx (XX) 2019/628 xxx xxx 21.&xxxx;xxxxx&xxxx;2021 x&xxxx;xxxxxxxxx x&xxxx;xxxxxxxxx xxxxxxxxx xx xxxxxxx xxxxxx xxxxxxxxx xxxxxxxxx x&xxxx;xxxxxxxx xxxxxxxxxx xxxxxxxx. |
|
(4) |
Xxxxxxxxx xxxxxxxx (XX) 2020/2235 xxxxxxx xxxxxxxxx xxxx xxxxxxxx xxxxxxxxxxx vydanými v tištěné xxxxxx, xxxxxxxxxxxxxx úředními xxxxxxxxxxx vydanými x&xxxx;xxxxxxx x&xxxx;xxxxxxxxx xx.&xxxx;39 odst. 1 xxxxxxxxxxx nařízení (EU) 2019/1715 x&xxxx;xxxxxxxx osvědčeními xxxxxxxx x&xxxx;xxxxxxx xxxxxx x&xxxx;xxxxxxxxxx v systému XXXXXX x&xxxx;xxxxxxxxxxx z tohoto xxxxxxx. Xxxxx xxxx uvedené xxxxxxxxx xxxxxxxx stanoví xxxxxxxx požadavky xx xxxxxx xxxxxxxxx xxx xxxxx do Xxxx, xxx se xxxxxxxxx xxxxxx kontroly xx xxxxxxxxxxxx xxxxxxxx kontroly x&xxxx;xxxxx vstupu do Xxxx. Xxx se xxxxxxx xxxxxx xxxxxxxxx xxx xxxxx xxxxxxxxx xxxxx a zajistil se xxxxxx x&xxxx;xxxxxx xxxxxxxxx xx osvědčování x&xxxx;xxxxxxxx xxxxxxxxxxx xxx vstup xx Unie xxxxxxxxxxx x&xxxx;xxxxxxxxxx xxxxxxxx (EU) 2020/2235, je vhodné xxxxxx článek 11 xxxxxxxxxxx xxxxxxxx (EU) 2019/1793. |
|
(5) |
Xxxxxx&xxxx;12 xxxxxxxxxxx nařízení (XX) 2019/1793 xxxxxxx, xx xxxxxxx xxxxxxx v přílohách X&xxxx;x&xxxx;XX budou x&xxxx;xxxxxx xxxxxxxxxxxxxx xxxx xxxxxx xxxxxxxxxx xxxxxxxxxxxxx s cílem xxxxxxxxx nové xxxxxxxxx xxxxxxxx se rizik x&xxxx;xxxxxxxxx x&xxxx;xxxxxx Xxxx. |
|
(6) |
Xxxxxx x&xxxx;xxxxxxxxx nedávných xxxxxxx x&xxxx;xxxxxxx xxxxxxxx xxxxxxxxxx xxxxxxxxxxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxxxx pro xxxxxxxxx a krmiva (RASFF), xxxxx xxx xxxxxx xxxxxxxxx (ES) x.&xxxx;178/2002, x&xxxx;xxxxxxxxx x&xxxx;xxxxxxxx xxxxxxxxxx xxxxxxxxxxx xxxxxxxxx xxxxx x&xxxx;xxxxxxxx x&xxxx;xxxxx xxxxxx xxx xxxxxxxxxxx xxxxxx xxxxxxxxx, že xxxxxxx x&xxxx;xxxxxxxxx X&xxxx;x&xxxx;XX xxxxxxxxxxx xxxxxxxx (XX) 2019/1793 xx xxxx být xxxxxxx. |
|
(7) |
Xxxxx xxxx, vzhledem x&xxxx;xxxxxxx nesouladu s příslušnými xxxxxxxxx stanovenými x&xxxx;xxxxxxxx xxxxxxxxxx Xxxx, pokud xxx x&xxxx;xxxxxxxxxxx xxxxxxxxxxx xxxxxxxxx při xxxxxxxx xxxxxxxxxx prováděných xxxxxxxxx xxxxx xxxxx prováděcího xxxxxxxx (EU) 2019/1793 x&xxxx;xxxx 2019 x&xxxx;x&xxxx;xxxxxx xxxxxxxx xxxx 2020, x&xxxx;x&xxxx;xxxxxxxx xxxxx oznámení x&xxxx;xxxxxxx RASFF během xxxxxx xxxxxx je xxxxxx zvýšit četnost xxxxxxx totožnosti a fyzických xxxxxxx x&xxxx;xxxxxxx xxxxx (Xxxxx nigrum) x&xxxx;Xxxxxxxx x&xxxx;20 na 50&xxxx;%. |
|
(8) |
Xxxxxxxx x&xxxx;xxxxxxx xxxxxxxxx s příslušnými xxxxxxxxx xxxxxxxxxxx v právních xxxxxxxxxx Xxxx, xxxxx xxx o kontaminaci xxxxxxx xxxxxxxxx xxxxxxxxx při xxxxxxxx kontrolách prováděných xxxxxxxxx státy xxxxx xxxxxxxxxxx xxxxxxxx (EU) 2019/1793 ve xxxxxx xxxxxxxx roku 2019 x&xxxx;x&xxxx;xxxxxx pololetí xxxx 2020, xx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxxxxx a fyzických xxxxxxx x&xxxx;xxxxxxx xxxx Xxxxxxxx (xxxx xxx xxxxxx) x&xxxx;Xxxxxxx x&xxxx;10 xx 20&xxxx;%. |
|
(9) |
Xxxxxxxx x&xxxx;xxxxxxx nesouladu x&xxxx;xxxxxxxxxxx požadavky stanovenými x&xxxx;xxxxxxxx xxxxxxxxxx Xxxx, xxxxx xxx x&xxxx;xxxxxxxxxxx xxxxxxxxxx xxxxxxxxx při xxxxxxxx xxxxxxxxxx xxxxxxxxxxx xxxxxxxxx státy xxxxx xxxxxxxxxxx nařízení (XX) 2019/1793 ve xxxxxx xxxxxxxx xxxx 2019 x&xxxx;x&xxxx;xxxxxx xxxxxxxx xxxx 2020, x&xxxx;x&xxxx;xxxxxxxx xxxxx xxxxxxxx x&xxxx;xxxxxxx RASFF xxxxx xxxxxxx xxxxxxxx xxxx 2020 je xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxxxxx x&xxxx;xxxxxxxxx xxxxxxx x&xxxx;xxxxxxxxxx xxxxxx x&xxxx;Xxxxx z 10 xx 50&xxxx;%. |
|
(10) |
Xxxxxx paprika (Xxxxxxxx xxxxxx) x&xxxx;Xxxxxxx xx xxx xxxxxxx v příloze X&xxxx;xxxxxxxxxxx xxxxxxxx (XX) 2019/1793 z důvodu xxxxxx xxxxxxxxxxx xxxxxxx xxxxxxxxx. X&xxxx;xxxxxxx xxxxxxx paprik xxxx Xxxxxxxx (xxxxxx xxx xxxxxxxx) x&xxxx;Xxxxxxx xxxxxxxxx xxxxx xxxxxxx xx xxxxxxx xxxxxxxx xxxxxxxxxx xxxxxxxxxxxxxxx xxxxxxx XXXXX x&xxxx;xxxxxx pololetí xxxx 2020 xx xxxxx xxxxxx rizik xxx xxxxxx xxxxxx x&xxxx;xxxxxxxx možné xxxxxxxxxxx xxxxxxx pesticidů, xxx xxxxxxxx xxxxxxxx xxxxxxxxxx xxxxxxxx xxxxxxx. Stávající xxxxxxx xxxxxxxx xx xxxxxx papriky (Capsicum xxxxxx) x&xxxx;Xxxxxxx by xxxxx xxxx být xxxxxxx tak, xxx xx xxxxxxxxxx xx xxxxxxx papriky xxxx Xxxxxxxx. |
|
(11) |
Xxxxx jde x&xxxx;xxxxxxxxxx xxxxxxx (xxxxxxxxx) x&xxxx;Xxxx xxxxxxxx x&xxxx;xxxxxxx I prováděcího xxxxxxxx (XX) 2019/1793 x&xxxx;xxxxxx rizika kontaminace xxxxxxx pesticidů a pokud xxx x&xxxx;xxxxxx xxxxxx x&xxxx;Xxxxxxx uvedené xx xxxxxxx xxxxxxx z důvodu xxxxxx kontaminace xxxxxxxxxxxx X, z dostupných xxxxxxxxx xx xxxxx xxxxxxxx xxxx 2019 x&xxxx;xxxxx xxxxxxxx roku 2020 xxxxxxx xxxxxxx uspokojivý xxxxxx souladu x&xxxx;xxxxxxxxxxx xxxxxxxxx xxxxxxxxxxx v právních xxxxxxxxxx Unie. Vzhledem x&xxxx;xxxx, že xxxxxxxx xxxxxx xxxxxxxx xxx xxxxx xxxxxx u uvedených xxxxxxx xxxxxxxxxx, měly xx být xxxxxxx x&xxxx;xxxxxxx I prováděcího xxxxxxxx (XX) 2019/1793 xxxxxxxx xx xxxxxxxxx komodit xxxxxxx. |
|
(12) |
Xxxxx xxx o podzemnici xxxxxxx x&xxxx;Xxxxxxxx uvedenou x&xxxx;xxxxxxx XX prováděcího xxxxxxxx (XX) 2019/1793 x&xxxx;xxxxxx xxxxxx kontaminace xxxxxxxxxx, xxxxx xx xxxxxxx četnosti xxxxxxx xxxxxxxxx s příslušnými xxxxxxxxx xxxxxxxx předpisů Xxxx, xxx byly xxxxxxxx xxx xxxxxxxx kontrolách xxxxxxxxxxx xxxxxxxxx xxxxx xx xxxxxx xxxxxxxx xxxx 2019, a v prvním xxxxxxxx xxxx 2020 xxxxxxx xx xxxxx xxxxxx. Xx xxxxx xxxxxx xxxxxxxxx položku xxxxxxxx xx podzemnice xxxxxx z Brazílie z přílohy XX xxxxxxxxxxx xxxxxxxx (XX) 2019/1793 x&xxxx;xxxxxxx xx xx přílohy X&xxxx;xxxxxxxxx xxxxxxxxxxx nařízení x&xxxx;xxxxxxxx xxxxxxx xxxxxxx xxxxxxxxxx x&xxxx;xxxxxxxxx xxxxxxx xx 10&xxxx;%. |
|
(13) |
Xxxxx xxx x&xxxx;xxxxxxxxxx olejnou z Číny xxxxxxxx x&xxxx;xxxxxxx II xxxxxxxxxxx xxxxxxxx (XX) 2019/1793 x&xxxx;xxxxxx rizika xxxxxxxxxxx aflatoxiny, xxxxx xx xxxxxxx xxxxxxxx xxxxxxx xxxxxxxxx x&xxxx;xxxxxxxxxxx xxxxxxxxx právních xxxxxxxx Xxxx, jež xxxx xxxxxxxx xxx xxxxxxxx xxxxxxxxxx xxxxxxxxxxx xxxxxxxxx xxxxx xx xxxxxx xxxxxxxx xxxx 2019 x&xxxx;x&xxxx;xxxxxx xxxxxxxx xxxx 2020. Je xxxxx xxxxxx odstranit xxxxxxx xxxxxxxx xx podzemnice xxxxxx x&xxxx;Xxxx x&xxxx;xxxxxxx XX xxxxxxxxxxx xxxxxxxx (XX) 2019/1793 a zařadit xx xx xxxxxxx X&xxxx;xxxxxxxxx xxxxxxxxxxx xxxxxxxx x&xxxx;xxxxxxxx xxxxxxx kontrol xxxxxxxxxx a fyzických xxxxxxx xx 10 %. X&xxxx;xxxxxxx xx xxxxx obchodu x&xxxx;xxxxx xxxxxxxxx xx xxxx xxxxxxx dostatečná x&xxxx;xxxxxxxxx odpovídající xxxxxx xxxxxxxxx. |
|
(14) |
Xxxxx xxx o lískové xxxxxx x&xxxx;Xxxxxxx xxxxxxx x&xxxx;xxxxxxx XX prováděcího xxxxxxxx (XX) 2019/1793 x&xxxx;xxxxxx rizika xxxxxxxxxxx xxxxxxxxxx, xxxxx ke xxxxxxx četnosti xxxxxxx xxxxxxxxx s příslušnými xxxxxxxxx xxxxxxxx předpisů Xxxx, xxx xxxx zjištěny xxx xxxxxxxx xxxxxxxxxx xxxxxxxxxxx xxxxxxxxx státy xx xxxxxx pololetí xxxx 2019 a v prvním xxxxxxxx xxxx 2020. Xx xxxxx vhodné xxxxxxxxx položku xxxxxxxx xx xxxxxxxxx xxxxxx x&xxxx;Xxxx z přílohy XX xxxxxxxxxxx xxxxxxxx (EU) 2019/1793 x&xxxx;xxxxxxx xx xx přílohy I uvedeného xxxxxxxxxxx nařízení x&xxxx;xxxxxxxx xxxxxxx xxxxxxx xxxxxxxxxx x&xxxx;xxxxxxxxx xxxxxxx xx 5&xxxx;%. X&xxxx;xxxxxxx na xxxxx obchodu s touto xxxxxxxxx xx tato xxxxxxx dostatečná x&xxxx;xxxxxxxxx xxxxxxxxxxxx xxxxxx xxxxxxxxx. |
|
(15) |
Xxxxxxxxx xxxxxxxxxx xxxxx xxxxx xxxxxxxxxx (Piper betle) xxxx x&xxxx;xxxx xxxxxxxxxxx xxxxxxxxxxx nebo zasílané x&xxxx;Xxxxxxxxxx xxxx xxxxxxx x&xxxx;xxxxxxx XXx prováděcího xxxxxxxx (EU) 2019/1793 x&xxxx;xxxxxx rizika kontaminace xxxxxxxxxxx. Xxxxx xxxxxx xxxxxxx xx Xxxx xx xxxxx xx xxxxxx 2014 xxxxxxx. Xxxxxxxxx xxxxxxx xxxxxxx xxxxxx xxx, xx xxx 27.&xxxx;xxxxxxxx&xxxx;2020 xxxxxxxxx xxxx akční xxxx xxxxxxxxxx xxxxxxxx xxxxxxxx xx všech xxxx xxxxxxxxx řetězce x&xxxx;Xxxxxx xxx xxxxxxxxxxx jako xxxxxxxxxx. X&xxxx;xxxxxxxxxx xx xxxx xxxxxxxxx xx xxxxx vhodné xxxxxxxxx xxxxxxx xxxxxxxx se xxxxxxxx xxxxxxxxxxxx listy xxxxx xxxxxxxxxx (Piper xxxxx) xxxx z nich xxxxxxxxxxxxx pocházejících xxxx xxxxxxxxxx x&xxxx;Xxxxxxxxxx x&xxxx;xxxxxxx XXx xxxxxxxxxxx xxxxxxxx (XX) 2019/1793 x&xxxx;xxxxxxx xx do přílohy XX xxxxxxxxx xxxxxxxxxxx xxxxxxxx a stanovit xxxxxxx xxxxxxx xxxxxxxxxx x&xxxx;xxxxxxxxx xxxxxxx na 50 %. |
|
(16) |
Aby xxxx xxxxxxxxx účinná xxxxxxx xxxx možnými xxxxxxxxxxx xxxxxx vyplývajícími x&xxxx;xxxxxxxxxxxxxxx nebo xxxxxxxx xxxxxxxxxxx sezamových xxxxx, xx sloupcích xxxxxxxxxxxx xx „xxx XX“ x&xxxx;xxxxxxxxx x&xxxx;xxxxxxxxx X&xxxx;x&xxxx;XX xxxxxxxxxxx xxxxxxxx (EU) 2019/1793 xx xxx xx xxx xx xxxxx x&xxxx;xxxxxxx xx „xxxxxxxx xxxxxx (xxxxxxxxx)“ xxxxxxx xxx XX xxx xxxxxxx sezamová xxxxxx. |
|
(17) |
X&xxxx;xxxxx II vzorového xxxxxxxx xxxxxxxxx x&xxxx;xxxxxxx XX xxxxxxxxxxx xxxxxxxx (XX) 2019/1793 se xxxxxxx zdravotní informace, xxx xxxxxxxxxxx xxxxxxx xxxxxxxx xxx xxxxxxxxxx xxxxxxxxx. X&xxxx;xxxxx zajištění xxxxxx xxxxxxx xx xxxx xxx xxxxxxxxx, xx xxxxxxxxx xxxxxxxxx xxxxxxxx se xxxxxxxx xxxx xxxxx xxxxxx xxx živočišného původu xxxxx xxxxxxxxx xxxx xxx jedno osvědčení, xxxxx xx takové xxxxxxxxx xxxxxxx xxxxx xx.&xxxx;11 xxxx.&xxxx;1 xxxxxxxxxxx xxxxxxxx (XX) 2019/1793 xx xxxxxxx x&xxxx;xxxxxxxx XX xxxxxxxxx prováděcího xxxxxxxx. |
|
(18) |
Xxxxxxxxx xxxxxxxx (XX) 2019/1793 xx proto xxxx být odpovídajícím xxxxxxxx xxxxxxx. V zájmu xxxxxxxxx xxxxxxxxxxxxxxx x&xxxx;xxxxxxxxxxxx xx xxxxxx xxxxxxxx xxxxxxx X, II, XXx x&xxxx;XX xxxxxxxxx xxxxxxxxxxx xxxxxxxx v jejich xxxxxxxx. |
|
(19) |
Xxxxxxxx k tomu, xx xx prováděcí xxxxxxxx (XX) 2020/2235 xxxxxxx xxx dne 21.&xxxx;xxxxx&xxxx;2021, xxx by se xx.&xxxx;1 xxxx.&xxxx;1 xxxxxx xxxxxxxx xxxxxx xxxxxx xx xxxxxxxxx data. |
|
(20) |
Opatření xxxxxxxxx tímto xxxxxxxxx xxxx x&xxxx;xxxxxxx xx xxxxxxxxxxx Xxxxxxx xxxxxx xxx rostliny, xxxxxxx, xxxxxxxxx a krmiva, |
PŘIJALA XXXX XXXXXXXX:
Xxxxxx&xxxx;1
Xxxxx xxxxxxxxxxx nařízení (XX) 2019/1793
Prováděcí nařízení (XX) 2019/1793 se xxxx xxxxx:
|
1) |
Xxxxxx&xxxx;11 xx xxxxxxxxx tímto: „Článek 11 Úřední xxxxxxxxx 1.&xxxx;&xxxx;&xxxx;Xxxxx xxxxxxx potravin x&xxxx;xxxxx xxxxxxxxx x&xxxx;xxxxxxx XX xxxx být xxxxxxxxxxx xxxxxxx xxxxxxxxxx x&xxxx;xxxxxxx xx xxxxxx uvedeným x&xxxx;xxxxxxx XX (xxxx xxx „xxxxxx osvědčení“). 2. Úřední xxxxxxxxx xxxx xxxxxxxx xxxx požadavky:
3. Odchylně xx xxxx.&xxxx;2 xxxx. x) může členský xxxx souhlasit x&xxxx;xxx, xxx byla úřední xxxxxxxxx vyhotovena x&xxxx;xxxxx xxxxxxx xxxxxx Xxxx x, je-li xx xxxxxxxx, opatřena ověřeným xxxxxxxxx. 4.&xxxx;&xxxx;&xxxx;Xxxxxx a razítko jiné xxx xxxxxxxx xxxx xxxxxxxxx, xx xxx xxxxxxxx odst. 2 xxxx. x), xxxx x&xxxx;xxxx xxxxx xxx barva xxxxx. 5.&xxxx;&xxxx;&xxxx;Xxxxxxxxxx xxxx.&xxxx;2 xxxx. x) až g) x&xxxx;xxxxxxxx 4 se xxxxxxxxx na xxxxxxxxxxxx xxxxxx xxxxxxxxx xxxxxx x&xxxx;xxxxxxx s požadavky čl. 39 xxxx.&xxxx;1 xxxxxxxxxxx xxxxxxxx Xxxxxx (XX) 2019/1715 (*1). 6.&xxxx;&xxxx;&xxxx;Xxxxxxxxxx xxxx.&xxxx;2 písm. x), x) a f) se xxxxxxxxx xx xxxxxx xxxxxxxxx xxxxxx x&xxxx;xxxxxxx xxxxxx x&xxxx;xxxxxxxx x&xxxx;xxxxxxx XXXXXX x&xxxx;x&xxxx;xxxxxx xxxxxxx xxxxxxxxx. 7.&xxxx;&xxxx;&xxxx;Xxxxxxxxx xxxxxx xxxxx xxxxx xxxxxxxx xxxxxx xxxxxxxxx xxxxx x&xxxx;xxxxxxx x&xxxx;xxxxxxxx stanovenými v článku 6 xxxxxxxxxxx xxxxxxxx Xxxxxx (XX) 2020/2235 (*2). 8.&xxxx;&xxxx;&xxxx;Xxxxxx xxxxxxxxx xx xxxxxx xxxxx xxxxxxxx xxxxxxxxx x&xxxx;xxxxxxx XX. (*1)&xxxx;&xxxx;Xxxxxxxxx xxxxxxxx Komise (XX) 2019/1715 xx xxx 30.&xxxx;xxxx&xxxx;2019, kterým xx stanoví xxxxxxxx xxx xxxxxxxxx xxxxxxx xxx xxxxxx informací x&xxxx;xxxxxxxx xxxxxxxxxx x&xxxx;xxxx xxxxxxxxxxx složek („xxxxxxxx x&xxxx;XXXXX“) (Úř. věst. L 261, 14.10.2019, s. 37)." (*2)&xxxx;&xxxx;Xxxxxxxxx xxxxxxxx Xxxxxx (EU) 2020/2235 xx xxx 16.&xxxx;xxxxxxxx&xxxx;2020, xxxxxx xx xxxxxxx xxxxxxxxx xxxxxxxx k nařízením Xxxxxxxxxx parlamentu x&xxxx;Xxxx (XX) 2016/429 x&xxxx;(XX) 2017/625, pokud xxx x&xxxx;xxxxxxx veterinární osvědčení, xxxxxxx xxxxxx osvědčení x&xxxx;xxxxxxx xxxxxxxxxxx/xxxxxx xxxxxxxxx xxx xxxxx xxxxxxx xxxxxxxx xxxxxxxxx xxxxxx x&xxxx;xxxxx do Unie x&xxxx;xxxxxx přemísťování x&xxxx;xxxxx Xxxx x&xxxx;x&xxxx;xxxxxx xxxxxxxxxxx xxxxxxxx xx těchto xxxxxxxxx, a kterým xx xxxxxxx nařízení (XX) x.&xxxx;599/2004, xxxxxxxxx nařízení (XX) x.&xxxx;636/2014 x&xxxx;(XX) 2019/628, směrnice 98/68/XX x&xxxx;xxxxxxxxxx 2000/572/ES, 2003/779/ES x&xxxx;2007/240/XX (Úř. věst. L 442, 30.12.2020, s. 1).“." |
|
2) |
Xxxxxxx X, XX, XXx x&xxxx;XX xx xxxxxxxxx zněním xxxxxxxx v příloze xxxxxx xxxxxxxx. |
Xxxxxx&xxxx;2
Xxxxx x&xxxx;xxxxxxxx a použitelnost
Toto xxxxxxxx xxxxxxxx x&xxxx;xxxxxxxx xxxxxxxx dnem xx xxxxxxxxx x&xxxx;Xxxxxxx xxxxxxxx Xxxxxxxx xxxx.
Xxxxxxxxxx xx.&xxxx;1 xxxx.&xxxx;1 se xxxxxxx xxx xxx 21. dubna 2021.
Xxxx nařízení xx xxxxxxx x&xxxx;xxxxx xxxxxxx x&xxxx;xxxxx použitelné xx xxxxx xxxxxxxxx státech.
V Bruselu xxx 14. dubna 2021.
Xx Xxxxxx
xxxxxxxxxxx
Xxxxxx XXX DER XXXXX
(1)&xxxx;&xxxx;Xx. xxxx. X&xxxx;31, 1.2.2002, x.&xxxx;1.
(2)&xxxx;&xxxx;Xx. xxxx. X&xxxx;95, 7.4.2017, x.&xxxx;1.
(3)&xxxx;&xxxx;Xxxxxxxxx xxxxxxxx Xxxxxx (XX) 2019/1793 xx xxx 22.&xxxx;xxxxx&xxxx;2019 o dočasném xxxxxxxxxxxxx xxxxxxxx xxxxxxx x&xxxx;xxxxxxxxxxx xxxxxxxx upravujících xxxxx xxxxxxxx zboží x&xxxx;xxxxxxxx xxxxxxx xxxx do Xxxx, xxxxxx xx xxxxxxxxx xxxxxxxx Xxxxxxxxxx xxxxxxxxxx a Rady (EU) 2017/625 x&xxxx;(XX) x.&xxxx;178/2002 x&xxxx;xxxxxx xx xxxxxxx xxxxxxxx Xxxxxx (XX) x.&xxxx;669/2009, (XX) č. 884/2014, (XX) 2015/175, (EU) 2017/186 a (EU) 2018/1660 (Xx. xxxx. X&xxxx;277, 29.10.2019, s. 89).
(4) Prováděcí xxxxxxxx Xxxxxx (XX) 2019/1715 xx dne 30.&xxxx;xxxx&xxxx;2019, xxxxxx xx stanoví xxxxxxxx pro xxxxxxxxx xxxxxxx xxx xxxxxx xxxxxxxxx x&xxxx;xxxxxxxx kontrolách x&xxxx;xxxx xxxxxxxxxxx xxxxxx („xxxxxxxx x&xxxx;XXXXX“) (Úř. xxxx. X&xxxx;261, 14.10.2019, x.&xxxx;37).
(5)&xxxx;&xxxx;Xxxxxxxxx nařízení Komise (XX) 2019/628 ze xxx 8. dubna 2019 o vzorových xxxxxxxx xxxxxxxxxxx xxx xxxxxxx xxxxxxx x&xxxx;xxxxx x&xxxx;x&xxxx;xxxxx xxxxxxxx (XX) x.&xxxx;2074/2005 x&xxxx;xxxxxxxxxxx xxxxxxxx (XX) 2016/759, pokud xxx x&xxxx;xxxx vzorová xxxxxxxxx (Xx. věst. X&xxxx;131, 17.5.2019, s. 101).
(6) Prováděcí xxxxxxxx Xxxxxx (XX) 2020/2235 xx xxx 16.&xxxx;xxxxxxxx&xxxx;2020, xxxxxx xx xxxxxxx prováděcí pravidla x&xxxx;xxxxxxxxx Xxxxxxxxxx xxxxxxxxxx x&xxxx;Xxxx (XX) 2016/429 x&xxxx;(XX) 2017/625, xxxxx xxx x&xxxx;xxxxxxx veterinární xxxxxxxxx, xxxxxxx xxxxxx xxxxxxxxx x&xxxx;xxxxxxx xxxxxxxxxxx/xxxxxx xxxxxxxxx xxx xxxxx xxxxxxx xxxxxxxx kategorií xxxxxx x&xxxx;xxxxx do Xxxx x&xxxx;xxxxxx xxxxxxxxxxxx x&xxxx;xxxxx Xxxx a o úřední xxxxxxxxxxx xxxxxxxx xx xxxxxx osvědčení, x&xxxx;xxxxxx xx xxxxxxx xxxxxxxx (XX) č. 599/2004, prováděcí xxxxxxxx (XX) x.&xxxx;636/2014 x&xxxx;(XX) 2019/628, směrnice 98/68/XX x&xxxx;xxxxxxxxxx 2000/572/XX, 2003/779/XX x&xxxx;2007/240/XX (Úř. xxxx. X&xxxx;442, 30.12.2020, x.&xxxx;1).
PŘÍLOHA I
Potraviny a krmiva xxxxxx xxx xxxxxxxxxxx xxxxxx, které xxxxxxxxx x&xxxx;xxxxxxxx xxxxxxx xxxx x&xxxx;xxxxxxxxx dočasnému zintenzivnění xxxxxxxx xxxxxxx na xxxxxxxxxxxx xxxxxxxx kontroly x&xxxx;xx kontrolních xxxxxxx
|
Xxxxx |
Xxxxxxxxx x&xxxx;xxxxxx (xxxxxxxxx xxxxxxx) |
Xxx KN &xxxx;(1) |
Xxxxxxx TARIC |
Země xxxxxx |
Xxxxxx |
Xxxxxxx xxxxxxx xxxxxxxxxx x&xxxx;xxxxxxxxx xxxxxxx (%) |
||||||
|
1 |
|
|
Bolívie (XX) |
Xxxxxxxxxx |
50 |
|||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|||||||||||
(Xxxxxxxxx x&xxxx;xxxxxx) |
|
20 |
||||||||||
|
2 |
(Xxxxxxxxx – xxxxxxxx xxx xxxxxxx) |
xx&xxxx;0904&xxxx;11&xxxx;00 |
10 |
Xxxxxxxx (XX) |
Xxxxxxxxx &xxxx;(2) |
50 |
||||||
|
3 |
|
|
Xxxxxxxx (XX) |
Xxxxxxxxxx |
10 |
|||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|||||||||||
(Xxxxxxxxx a krmiva) |
|
20 |
||||||||||
|
4 |
|
|
Čína (XX) |
Xxxxxxxxxx |
10 |
|||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|||||||||||
(Xxxxxxxxx x&xxxx;xxxxxx) |
|
20 |
||||||||||
|
5 |
Xxxxxx xxxxxxx (Xxxxxxxx xxxxxx) (Xxxxxxxxx – xxxxxx xxxx xxxxx) |
xx&xxxx;0904&xxxx;22&xxxx;00 |
11 |
Xxxx (XX) |
Xxxxxxxxx (6) |
20 |
||||||
|
6 |
Čaj, xxx xxxxxxxxxxxxx (Xxxxxxxxx) |
0902 |
Čína (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(3) &xxxx;(7) |
20 |
|||||||
|
7 |
Xxxxx (Xxxxxxx melongena) (Potraviny – čerstvé xxxx xxxxxxxx) |
0709&xxxx;30&xxxx;00 |
Xxxxxxxxxxxx xxxxxxxxx (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(3) |
20 |
|||||||
|
8 |
|
|
Xxxxxxxxxxxx xxxxxxxxx (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(3) &xxxx;(8) |
50 |
|||||||
|
|
20 |
||||||||||
|
xx&xxxx;0710&xxxx;80&xxxx;59 |
20 |
|||||||||||
(Xxxxxxxxx – xxxxxxx, xxxxxxxx xxxx xxxxxxxx) |
|
10 |
||||||||||
|
ex 0710 22 00 |
10 |
|||||||||||
|
9 |
|
|
Xxxxx (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(3) &xxxx;(9) |
20 |
|||||||
(Xxxxxxxxx – xxxxxxx, xxxxxxxx xxxx zmrazené) |
|
20 |
||||||||||
|
xx&xxxx;0710&xxxx;80&xxxx;59 |
20 |
|||||||||||
|
10 |
Xxxxxxxx xxxxxx (Xxxxxxxxx) |
|
Xxxxxxx (XX) |
Xxxxxxxxx (2) |
50 |
|||||||
|
40 |
|||||||||||
|
40 |
|||||||||||
|
11 |
|
|
Xxxxxx (GE) |
Aflatoxiny |
50 |
|||||||
|
|
|||||||||||
|
|
40 |
||||||||||
(Xxxxxxxxx) |
|
30 |
||||||||||
|
xx&xxxx;2008&xxxx;19&xxxx;95 ; |
20 |
|||||||||||
|
ex 2008 19 99 |
30 |
|||||||||||
|
12 |
Palmový xxxx (Xxxxxxxxx) |
1511&xxxx;10&xxxx;90 ; 1511&xxxx;90&xxxx;11 ; |
Xxxxx (XX) |
Xxxxxxx Xxxxx &xxxx;(10) |
50 |
|||||||
|
xx&xxxx;1511&xxxx;90&xxxx;19 ; |
90 |
|||||||||||
|
1511&xxxx;90&xxxx;99 |
||||||||||||
|
13 |
Xxxxx curry (Xxxxxxx/Xxxxxxx xxxxxxxx) (Xxxxxxxxx – xxxxxxx, xxxxxxxx, xxxxxxxx xxxx xxxxxx) |
xx&xxxx;1211&xxxx;90&xxxx;86 |
10 |
Xxxxx (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(3) &xxxx;(11) |
50 |
||||||
|
14 |
Xxxx (Xxxxxxxxx – xxxxxxx, xxxxxxxx xxxx zmrazené) |
xx&xxxx;0709&xxxx;99&xxxx;90 ; |
20 |
Xxxxx (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(3) (12) |
10 |
||||||
|
ex 0710 80 95 |
30 |
|||||||||||
|
15 |
Fazole (Xxxxx xxx., Xxxxxxxxx xxx.) (Xxxxxxxxx – xxxxxxx xxxx xxxxxxxx) |
0708&xxxx;20 |
Xxxx (XX) |
Xxxxxxx pesticidů (3) |
10 |
|||||||
|
16 |
Miřík celer (Xxxxx xxxxxxxxxx) (Xxxxxxxxx – xxxxxxx, xxxxxxx xxxx xxxxxxxx) |
ex 0709 40 00 |
20 |
Kambodža (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(3) (13) |
50 |
||||||
|
17 |
Fazole (Vigna xxxxxxxxxxx xxx. xxxxxxxxxxxxx, Xxxxx unguiculata xxx. xxxxxxxxxxx) (Xxxxxxxxx – xxxxxxxx, xxxxxxx, xxxxxxxx xxxx xxxxxxxx) |
xx&xxxx;0708&xxxx;20&xxxx;00 ; |
10 |
Xxxxxxxx (XX) |
Xxxxxxx pesticidů &xxxx;(3) &xxxx;(14) |
50 |
||||||
|
xx&xxxx;0710&xxxx;22&xxxx;00 |
10 |
|||||||||||
|
18 |
Xxxxxxx (Brassica rapa xxx. rapa) (Potraviny – xxxxxxxxxx xxxx konzervované x&xxxx;xxxx nebo xxxxxxxx xxxxxx) |
xx&xxxx;2001&xxxx;90&xxxx;97 |
11; 19 |
Xxxxxxx (XX) |
Xxxxxxxx X |
50 |
||||||
|
19 |
Xxxxxxx (Xxxxxxxx xxxx xxx. xxxx) (Xxxxxxxxx – xxxxxxxxxx xxxx xxxxxxxxxxxx xx xxxxxx xxxxxx nebo xxxxxxxx xxxxxxxxx, xxxxxxxxxx) |
xx&xxxx;2005&xxxx;99&xxxx;80 |
93 |
Xxxxxxx (XX) |
Xxxxxxxx X |
50 |
||||||
|
20 |
Xxxxxxx (Xxxxxxxx xxx.) (xxxxxx xxxx xxxx xxx xxxxxx) (Xxxxxxxxx – xxxxxx, xxxxxxx, xxxxxx xxxx xxxxx) |
0904 21 10 ; |
Xxx Lanka (XX) |
Xxxxxxxxxx |
50 |
|||||||
|
xx&xxxx;0904&xxxx;21&xxxx;90 ; |
20 |
|||||||||||
|
xx&xxxx;0904&xxxx;22&xxxx;00 ; |
11; 19 |
|||||||||||
|
xx&xxxx;2005&xxxx;99&xxxx;10 ; |
10; 90 |
|||||||||||
|
xx&xxxx;2005&xxxx;99&xxxx;80 |
94 |
|||||||||||
|
21 |
|
|
Xxxxxxxxxx (XX) |
Xxxxxxxxxx |
50 |
|||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|||||||||||
(Potraviny x&xxxx;xxxxxx) |
|
20 |
||||||||||
|
22 |
Xxxxxxxxx (Artocarpus heterophyllus) (Potraviny – čerstvé) |
xx&xxxx;0810&xxxx;90&xxxx;20 |
20 |
Xxxxxxxx (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(3) |
20 |
||||||
|
23 |
Xxxxxxxx xxxxxx (Xxxxxxxxx) |
|
Nigérie (XX) |
Xxxxxxxxx &xxxx;(2) |
50 |
|||||||
|
40 |
|||||||||||
|
40 |
|||||||||||
|
24 |
Xxxxx xxxxxx (Xxxxxxxxx) |
0910&xxxx;91&xxxx;10 ; 0910&xxxx;91&xxxx;90 |
Xxxxxxxx (XX) |
Xxxxxxxxxx |
50 |
|||||||
|
25 |
Xxxxxxxxx (egusi, Xxxxxxxxx spp.) xxxxx x&xxxx;xxxxxxxx x&xxxx;xxxx xxxxxxxx (Xxxxxxxxx) |
xx&xxxx;1207&xxxx;70&xxxx;00 ; |
10 |
Xxxxxx Leone (XX) |
Xxxxxxxxxx |
50 |
||||||
|
xx&xxxx;1208&xxxx;90&xxxx;00 ; |
10 |
|||||||||||
|
xx&xxxx;2008&xxxx;99&xxxx;99 |
50 |
|||||||||||
|
26 |
|
|
Xxxxxxx (SN) |
Aflatoxiny |
50 |
|||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|||||||||||
(Xxxxxxxxx x&xxxx;xxxxxx) |
|
20 |
||||||||||
|
27 |
Xxxxxxx (Xxxxxxxx xxxx xxx. rapa) (Potraviny – xxxxxxxxxx nebo xxxxxxxxxxxx x&xxxx;xxxx xxxx kyselině xxxxxx) |
ex 2001 90 97 |
11; 19 |
Xxxxx (XX) |
Xxxxxxxx X |
50 |
||||||
|
28 |
Xxxxxxx (Xxxxxxxx xxxx xxx. rapa) (Potraviny – xxxxxxxxxx xxxx xxxxxxxxxxxx xx slaném xxxxxx xxxx xxxxxxxx xxxxxxxxx, nezmrazené) |
xx&xxxx;2005&xxxx;99&xxxx;80 |
93 |
Xxxxx (XX) |
Xxxxxxxx X |
50 |
||||||
|
29 |
Xxxxxxx (xxxx xxx xxxxxx) (Capsicum xxx.) (Xxxxxxxxx – xxxxxxx, xxxxxxxx xxxx zmrazené) |
xx&xxxx;0709&xxxx;60&xxxx;99 ; |
20 |
Xxxxxxx (TH) |
Rezidua xxxxxxxxx &xxxx;(3) &xxxx;(15) |
20 |
||||||
|
xx&xxxx;0710&xxxx;80&xxxx;59 |
20 |
|||||||||||
|
30 |
|
|
Xxxxxxx (XX) |
Xxxxxxxxxx |
5 |
|||||||
|
|
|||||||||||
|
|
70 |
||||||||||
|
xx&xxxx;0813&xxxx;50&xxxx;91 ; |
70 |
|||||||||||
|
xx&xxxx;0813&xxxx;50&xxxx;99 |
70 |
|||||||||||
|
|
70 |
||||||||||
|
ex 2007 10 99 ; |
40 |
|||||||||||
|
ex 2007 99 39 ; |
05; 06 |
|||||||||||
|
xx&xxxx;2007&xxxx;99&xxxx;50 ; |
33 |
|||||||||||
|
ex 2007 99 97 |
23 |
|||||||||||
|
|
30 |
||||||||||
|
ex 2008 19 19 ; |
30 |
|||||||||||
|
xx&xxxx;2008&xxxx;19&xxxx;92 ; |
30 |
|||||||||||
|
xx&xxxx;2008&xxxx;19&xxxx;95 ; |
20 |
|||||||||||
|
xx&xxxx;2008&xxxx;19&xxxx;99 ; |
30 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;12 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;14 ; |
15 |
|||||||||||
|
ex 2008 97 16 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;18 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;32 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;34 ; |
15 |
|||||||||||
|
ex 2008 97 36 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;38 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;51 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;59 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;72 ; |
15 |
|||||||||||
|
ex 2008 97 74 ; |
15 |
|||||||||||
|
ex 2008 97 76 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;78 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;92 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;93 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;94 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;96 ; |
15 |
|||||||||||
|
ex 2008 97 97 ; |
15 |
|||||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;98 |
15 |
|||||||||||
|
|
40 |
||||||||||
(Xxxxxxxxx) |
|
20 |
||||||||||
|
31 |
Xxxxxxxxxx (xxxxxx xxxxxxxxxx x&xxxx;xxxxxx); xxxxxxxxxxx, xxxxxxxx x&xxxx;xxxxxxx xxxxxxxxx xxxxxxx (Xxxxxxxxx – čerstvé xxxx xxxxxx) |
0805&xxxx;21 ; 0805&xxxx;22 ; 0805&xxxx;29 |
Xxxxxxx (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(3) |
5 |
|||||||
|
32 |
Xxxxxxxxx (Xxxxxxxxx – xxxxxxx nebo sušené) |
0805&xxxx;10 |
Xxxxxxx (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(3) |
10 |
|||||||
|
33 |
Xxxxxxxxx xxxxxx (Xxxxxxxxx – xxxxxxx xxxx xxxxxxxx) |
xx&xxxx;0810&xxxx;90&xxxx;75 |
30 |
Xxxxxxx (TR) |
Rezidua xxxxxxxxx &xxxx;(3) &xxxx;(16) |
20 |
||||||
|
34 |
(Xxxxxxxxx – xxxxxxx, xxxxxxxx xxxx zmrazené) |
|
Turecko (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(3) &xxxx;(17) |
10 |
|||||||
|
20 |
|||||||||||
|
xx&xxxx;0710&xxxx;80&xxxx;59 |
20 |
|||||||||||
|
35 |
Xxxxxxxxxxxx xxxx, xxxxxxxxx, rozemletá, rozloupnutá x&xxxx;xxxxxxxxx xxxxxxxxx xxxxx xxxxxx k uvedení xx xxx xxx xxxxxxxxx xxxxxxxxxxxx &xxxx;(18) &xxxx;(19) (Xxxxxxxxx) |
xx&xxxx;1212&xxxx;99&xxxx;95 |
20 |
Xxxxxxx (XX) |
Xxxxxx |
50 |
||||||
|
36 |
Xxxxxxx (jiné xxx sladké) (Capsicum xxx.) (Xxxxxxxxx – xxxxxxx, xxxxxxxx xxxx xxxxxxxx) |
xx&xxxx;0709&xxxx;60&xxxx;99 ; |
20 |
Xxxxxx (UG) |
Rezidua xxxxxxxxx &xxxx;(3) |
20 |
||||||
|
xx&xxxx;0710&xxxx;80&xxxx;59 |
20 |
|||||||||||
|
37 |
|
|
Xxxxxxx xxxxx xxxxxxxx (XX) |
Xxxxxxxxxx |
10 |
|||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|||||||||||
(Xxxxxxxxx a krmiva) |
|
20 |
||||||||||
|
38 |
|
|
Xxxxxxx xxxxx xxxxxxxx (XX) |
Xxxxxxxxxx |
10 |
|||||||
|
|
|||||||||||
|
|
20 |
||||||||||
|
xx&xxxx;2008&xxxx;19&xxxx;93 |
20 |
|||||||||||
|
39 |
|
|
Xxxxxxxxxx (UZ) |
Siřičitany &xxxx;(20) |
50 |
|||||||
(Xxxxxxxxx) |
|
|||||||||||
|
40 |
|
|
72 |
Vietnam (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(3) &xxxx;(21) |
50 |
||||||
|
|
20 |
||||||||||
|
|
30 |
||||||||||
(Xxxxxxxxx – xxxxxxx, čerstvé xxxx xxxxxxxx) |
|
40 |
||||||||||
|
41 |
Xxxx (Xxxxxxxxx – xxxxxxx, chlazené xxxx xxxxxxxx) |
xx&xxxx;0709&xxxx;99&xxxx;90 ; |
20 |
Xxxxxxx (VN) |
Rezidua pesticidů &xxxx;(3) &xxxx;(21) |
50 |
||||||
|
xx&xxxx;0710&xxxx;80&xxxx;95 |
30 |
|||||||||||
|
42 |
Xxxxxxx (jiné xxx xxxxxx) (Capsicum xxx.) (Xxxxxxxxx – xxxxxxx, xxxxxxxx nebo zmrazené) |
xx&xxxx;0709&xxxx;60&xxxx;99 ; |
20 |
Xxxxxxx (VN) |
Rezidua xxxxxxxxx &xxxx;(3) &xxxx;(21) |
50 |
||||||
|
xx&xxxx;0710&xxxx;80&xxxx;59 |
20 |
(1)&xxxx;&xxxx;X&xxxx;xxxxxxx, xx xx x&xxxx;xxxxxx xxxx KN vyžaduje xxxxxxxxx jen u některých xxxxxxxx, xxxxxxxx se xxx XX xxxxxxxxx „xx“.
(2)&xxxx;&xxxx;Xxxxx xxxxxx x&xxxx;xxxxxxx xx xxxxxxxx x&xxxx;xxxxxxx x&xxxx;xxxxxxx xxxxxx vzorků x&xxxx;xxxxxxxxxxxx xxxxxxxxxxxx xxxxxxxx xxxxxxxxxxx x&xxxx;xxxxxxx XXX xxxx 1 xxxx. x).
(3)&xxxx;&xxxx;Xxxxxxx xxxxxxx xxxx xxxxxxxxx, xxxxx xxxx xxxxxxx v kontrolním xxxxxxxx xxxxxxxx v souladu x&xxxx;xx.&xxxx;29 xxxx.&xxxx;2 xxxxxxxx Xxxxxxxxxx xxxxxxxxxx x&xxxx;Xxxx (XX) x.&xxxx;396/2005 xx xxx 23.&xxxx;xxxxx&xxxx;2005 x&xxxx;xxxxxxxxxxx limitech xxxxxxx xxxxxxxxx x&xxxx;xxxxxxxxxxx x&xxxx;xxxxxxxx xxxxxxxxxxx a živočišného xxxxxx x&xxxx;xx xxxxxx xxxxxxx x&xxxx;x&xxxx;xxxxx xxxxxxxx Xxxx 91/414/XXX (Úř. xxxx. X&xxxx;70, 16.3.2005, x.&xxxx;1), která xxxxx xxx xxxxxxxx xxxxxxxxxxxxxxxxx xxxxxxxx xxxxxxxxxx xx XX-XX x&xxxx;XX-XX (xxxxxxxxx, xxxxx mají xxx xxxxxxxxxxxx xxxxx x&xxxx;xxxxxxxxxx xxxxxxxxxxx xxxxxx xxxx xx xxxxxx xxxxxxx).
(4)&xxxx;&xxxx;Xxxxxxx xxxxxxxx.
(5)&xxxx;&xxxx;Xxxxxxx xxxxxxxx.
(6)&xxxx;&xxxx;Xxxxx vzorků x&xxxx;xxxxxxx xx xxxxxxxx x&xxxx;xxxxxxx x&xxxx;xxxxxxx xxxxxx xxxxxx a analytickými xxxxxxxxxxxx xxxxxxxx xxxxxxxxxxx x&xxxx;xxxxxxx XXX bodě 1 xxxx. x).
(7)&xxxx;&xxxx;Xxxxxxx xxxxxxxxxxxx.
(8)&xxxx;&xxxx;Xxxxxxx xxxxxxxx (xxxxxxx včetně xxxxxxxxxx obsahujících 2,4-xxxxxxxxxxxxxxxxxx xxxxxxx, xxxxxxxxx jako xxxxxxx), xxxxxxxxxxxxxx, dikofolu (xxxx izomerů x, x’ x&xxxx;x, x’) x&xxxx;xxxxxxxxxxxxxxx (xxxxxxxxxxxxxxx, xxxxxxxxx xxxx CS2, xxxxxx xxxxxx, mankozebu, xxxxxxxx, xxxxxxxxx, xxxxxxx x&xxxx;xxxxxx).
(9)&xxxx;&xxxx;Xxxxxxx xxxxxxxx (suma xxxxxxx x, x’ x&xxxx;x, x’), xxxxxxxxxxxx, xxxxxxx, xxxxxxxxxxx (xxxx prochlorazu x&xxxx;xxxx metabolitů obsahujících 2,4,6-xxxxxxxxxxxxxxxxx xxxxxxx, xxxxxxxxx xxxx prochloraz), xxxxxxxxx-xxxxxxx x&xxxx;xxxxxxxxx.
(10)&xxxx;&xxxx;Xxx účely této xxxxxxx xx „barvivy Xxxxx“ xxxxxx xxxx xxxxxxxx xxxxx: x) Xxxxx X&xxxx;(xxxxx XXX 842-07-9); xx) Sudan XX (číslo XXX 3118-97-6); xxx) Xxxxx XXX (xxxxx XXX 85-86-9); xx) xxxxxxxxx xxxxxx xxxxxx Xxxxx XX (číslo XXX 85-83-6).
(11)&xxxx;&xxxx;Xxxxxxx xxxxxxx.
(12)&xxxx;&xxxx;Xxxxxxx diafenthiuronu.
(13) Rezidua xxxxxxxxx.
(14)&xxxx;&xxxx;Xxxxxxx xxxxxxxxxxx.
(15)&xxxx;&xxxx;Xxxxxxx xxxxxxxxxxx (xxxx xxxxxxxxxxx x&xxxx;xxxx xxxx, xxxxxxxxx xxxx xxxxxxxxxx (xxxxxxxxxxxx)), xxxxxxxxxxx x&xxxx;xxxxxxxxx.
(16)&xxxx;&xxxx;Xxxxxxx xxxxxxxxxxx.
(17)&xxxx;&xxxx;Xxxxxxx xxxxxxxxxxxxxx, xxxxxxxxxxx (xxxx formetanátu x&xxxx;xxxx solí, xxxxxxxxx xxxx xxxxxxxxxx (xxxxxxxxxxxx)) x&xxxx;xxxxxxxxx-xxxxxxx.
(18)&xxxx;&xxxx;„Xxxxxxxxxxxx xxxxxxxx“ xxxxx xxxxxxxx x&xxxx;xxxxxxxx Evropského xxxxxxxxxx a Rady (ES) x.&xxxx;852/2004 xx xxx 29.&xxxx;xxxxx&xxxx;2004 o hygieně potravin (Xx. xxxx. X&xxxx;139, 30.4.2004, x.&xxxx;1).
(19)&xxxx;&xxxx;„Xxxxxxx xx xxx“ a „konečný spotřebitel“ xxxxx definice v nařízení Xxxxxxxxxx xxxxxxxxxx x&xxxx;Xxxx (XX) č. 178/2002 ze xxx 28. ledna 2002, xxxxxx xx xxxxxxx xxxxxx xxxxxx x&xxxx;xxxxxxxxx xxxxxxxxxxxxx xxxxx, xxxxxxx xx Xxxxxxxx xxxx xxx xxxxxxxxxx potravin a stanoví xxxxxxx týkající xx xxxxxxxxxxx xxxxxxxx (Xx. xxxx. X&xxxx;31, 1.2.2002, x.&xxxx;1).
(20)&xxxx;&xxxx;Xxxxxxxxxx metody: XX 1988-1:1998, EN 1988-2:1998 xxxx ISO 5522:1981.
(21)&xxxx;&xxxx;Xxxxxxx xxxxxxxxxxxxxxx (dithiokarbamáty, xxxxxxxxx xxxx CS2, xxxxxx xxxxxx, xxxxxxxxx, metiramu, xxxxxxxxx, xxxxxxx x&xxxx;xxxxxx), xxxxxxxxx x&xxxx;xxxxxxxxxx.
XXXXXXX XX
Xxxxxxxxx a krmiva x&xxxx;xxxxxxxx třetích xxxx xxxxxxxxxxx xxxxxxxxx xxxxxxxxx xxx xxxxx do Xxxx z důvodu xxxxxx xxxxxxxxxxx xxxxxxxxxx včetně xxxxxxxxxx, rezidui xxxxxxxxx, xxxxxxxxxxxxxxxxx a dioxiny x&xxxx;xxxxxxxxxxxxxxx xxxxxxxxxxx
1.&xxxx;&xxxx;&xxxx;Xxxxxxxxx x&xxxx;xxxxxx xxxxxx xxx xxxxxxxxxxx xxxxxx xxxxxxx x&xxxx;xx.&xxxx;1 odst. 1 xxxx. x) bodě x)
|
Xxxxx |
Xxxxxxxxx a krmiva (zamýšlené xxxxxxx) |
Xxx KN (1) |
Třídění XXXXX |
Xxxx xxxxxx |
Xxxxxx |
Xxxxxxx xxxxxxx xxxxxxxxxx x&xxxx;xxxxxxxxx xxxxxxx (%) |
||||
|
1 |
|
|
Xxxxxxxxx (XX) |
Xxxxxxxxxx |
5 |
|||||
|
|
|||||||||
|
|
|||||||||
|
|
|||||||||
|
|
|||||||||
(Xxxxxxxxx a krmiva) |
|
20 |
||||||||
|
2 |
|
|
Ázerbájdžán (XX) |
Xxxxxxxxxx |
20 |
|||||
|
|
|||||||||
|
|
70 |
||||||||
|
xx&xxxx;0813&xxxx;50&xxxx;91 ; |
70 |
|||||||||
|
xx&xxxx;0813&xxxx;50&xxxx;99 |
70 |
|||||||||
|
|
70 |
||||||||
|
xx&xxxx;2007&xxxx;10&xxxx;99 ; |
40 |
|||||||||
|
xx&xxxx;2007&xxxx;99&xxxx;39 ; |
05; 06 |
|||||||||
|
ex 2007 99 50 ; |
33 |
|||||||||
|
ex 2007 99 97 |
23 |
|||||||||
|
|
30 |
||||||||
|
xx&xxxx;2008&xxxx;19&xxxx;19 ; |
30 |
|||||||||
|
xx&xxxx;2008&xxxx;19&xxxx;92 ; |
30 |
|||||||||
|
xx&xxxx;2008&xxxx;19&xxxx;95 ; |
20 |
|||||||||
|
ex 2008 19 99 ; |
30 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;12 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;14 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;16 ; |
15 |
|||||||||
|
ex 2008 97 18 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;32 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;34 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;36 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;38 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;51 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;59 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;72 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;74 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;76 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;78 ; |
15 |
|||||||||
|
ex 2008 97 92 ; |
15 |
|||||||||
|
ex 2008 97 93 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;94 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;96 ; |
15 |
|||||||||
|
ex 2008 97 97 ; |
15 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;98 |
15 |
|||||||||
|
|
40 |
||||||||
(Xxxxxxxxx) |
|
20 |
||||||||
|
3 |
(Potraviny) |
ex 1404 90 00 &xxxx;(10) |
10 |
Xxxxxxxxx (XX) |
Xxxxxxxxx &xxxx;(6) |
50 |
||||
|
4 |
|
|
Brazílie (XX) |
Xxxxxxxxxx |
50 |
|||||
(Xxxxxxxxx) |
|
20 |
||||||||
|
xx&xxxx;0813&xxxx;50&xxxx;39 ; |
20 |
|||||||||
|
xx&xxxx;0813&xxxx;50&xxxx;91 ; |
20 |
|||||||||
|
xx&xxxx;0813&xxxx;50&xxxx;99 |
20 |
|||||||||
|
5 |
|
|
Egypt (XX) |
Xxxxxxxxxx |
20 |
|||||
|
|
|||||||||
|
|
|||||||||
|
|
|||||||||
|
|
|||||||||
(Xxxxxxxxx x&xxxx;xxxxxx) |
|
20 |
||||||||
|
6 |
|
|
Etiopie (ET) |
Aflatoxiny |
50 |
|||||
(Xxxxxxxxx – xxxxxx xxxxxx) |
|
|||||||||
|
7 |
|
|
Xxxxx (XX) |
Xxxxxxxxxx |
50 |
|||||
|
|
|||||||||
|
|
|||||||||
|
|
|||||||||
|
|
|||||||||
(Xxxxxxxxx a krmiva) |
|
20 |
||||||||
|
8 |
|
|
Xxxxxx (XX) |
Xxxxxxxxxx |
50 |
|||||
|
|
|||||||||
|
|
|||||||||
|
|
|||||||||
|
|
|||||||||
(Xxxxxxxxx x&xxxx;xxxxxx) |
|
20 |
||||||||
|
9 |
Xxxxxxxxx xxxxxx (Myristica xxxxxxxx) (Xxxxxxxxx – xxxxxx xxxxxx) |
0908&xxxx;11&xxxx;00 ; 0908&xxxx;12&xxxx;00 |
Xxxxxxxxx (ID) |
Aflatoxiny |
20 |
|||||
|
10 |
Listy xxxxx betelového (Xxxxx xxxxx X.) (Xxxxxxxxx) |
xx&xxxx;1404&xxxx;90&xxxx;00 |
10 |
Xxxxx (XX) |
Xxxxxxxxx &xxxx;(2) |
10 |
||||
|
11 |
Xxxxxxx (xxxxxx xxxx jiné xxx xxxxxx) (Capsicum spp.) (Potraviny – sušené, xxxxxxx, xxxxxx xxxx xxxxx) |
0904&xxxx;21&xxxx;10 ; |
Indie (XX) |
Xxxxxxxxxx |
20 |
|||||
|
xx&xxxx;0904&xxxx;22&xxxx;00 ; |
11; 19 |
|||||||||
|
xx&xxxx;0904&xxxx;21&xxxx;90 ; |
20 |
|||||||||
|
ex 2005 99 10 ; |
10; 90 |
|||||||||
|
xx&xxxx;2005&xxxx;99&xxxx;80 |
94 |
|||||||||
|
12 |
Xxxxxxxxx xxxxxx (Xxxxxxxxx fragrans) (Potraviny – xxxxxx koření) |
0908&xxxx;11&xxxx;00 ; 0908&xxxx;12&xxxx;00 |
Xxxxx (XX) |
Xxxxxxxxxx |
20 |
|||||
|
13 |
|
|
Indie (IN) |
Aflatoxiny |
50 |
|||||
|
|
|||||||||
|
|
|||||||||
|
|
|||||||||
|
|
|||||||||
(Xxxxxxxxx x&xxxx;xxxxxx) |
|
20 |
||||||||
|
14 |
Guarová xxxx (Xxxxxxxxx x&xxxx;xxxxxx) |
xx&xxxx;1302&xxxx;32&xxxx;90 |
10 |
Xxxxx (XX) |
Xxxxxxxxxxxxxxx x&xxxx;xxxxxxx &xxxx;(3) |
5 |
||||
|
15 |
Xxxxxxx (jiné xxx xxxxxx) (Xxxxxxxx xxx.) (Xxxxxxxxx – xxxxxxx, xxxxxxxx nebo zmrazené) |
xx&xxxx;0709&xxxx;60&xxxx;99 ; |
20 |
Indie (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(4) &xxxx;(5) |
10 |
||||
|
xx&xxxx;0710&xxxx;80&xxxx;59 |
20 |
|||||||||
|
16 |
Xxxxxxxx xxxxxx (Xxxxxxxxx) |
|
Xxxxx (XX) |
Xxxxxxxxx &xxxx;(6) Xxxxxxx pesticidů &xxxx;(4) &xxxx;(11) |
20 |
|||||
|
40 |
|||||||||
|
40 |
50 |
||||||||
|
17 |
|
|
Xxxx (XX) |
Xxxxxxxxxx |
50 |
|||||
|
|
|||||||||
|
|
60 |
||||||||
|
xx&xxxx;0813&xxxx;50&xxxx;91 ; |
60 |
|||||||||
|
xx&xxxx;0813&xxxx;50&xxxx;99 |
60 |
|||||||||
|
|
60 |
||||||||
|
xx&xxxx;2007&xxxx;10&xxxx;99 ; |
30 |
|||||||||
|
ex 2007 99 39 ; |
03; 04 |
|||||||||
|
xx&xxxx;2007&xxxx;99&xxxx;50 ; |
32 |
|||||||||
|
xx&xxxx;2007&xxxx;99&xxxx;97 |
22 |
|||||||||
|
|
20 |
||||||||
|
xx&xxxx;2008&xxxx;19&xxxx;93 ; |
20 |
|||||||||
|
ex 2008 97 12 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;14 ; |
19 |
|||||||||
|
ex 2008 97 16 ; |
19 |
|||||||||
|
ex 2008 97 18 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;32 ; |
19 |
|||||||||
|
ex 2008 97 34 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;36 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;38 ; |
19 |
|||||||||
|
ex 2008 97 51 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;59 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;72 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;74 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;76 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;78 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;92 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;93 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;94 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;96 ; |
19 |
|||||||||
|
ex 2008 97 97 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;98 ; |
19 |
|||||||||
(Xxxxxxxxx) |
|
50 |
||||||||
|
18 |
Melounová (xxxxx, Citrullus xxx.) xxxxx x&xxxx;xxxxxxxx z nich xxxxxxxx (Xxxxxxxxx) |
ex 1207 70 00 ; |
10 |
Xxxxxxx (XX) |
Xxxxxxxxxx |
50 |
||||
|
xx&xxxx;1208&xxxx;90&xxxx;00 ; |
10 |
|||||||||
|
ex 2008 99 99 |
50 |
|||||||||
|
19 |
Papriky (xxxx xxx sladké) (Capsicum xxx.) (Xxxxxxxxx – xxxxxxx, xxxxxxxx xxxx zmrazené) |
xx&xxxx;0709&xxxx;60&xxxx;99 ; |
20 |
Xxxxxxxx (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(4) |
20 |
||||
|
xx&xxxx;0710&xxxx;80&xxxx;59 |
20 |
|||||||||
|
20 |
|
|
Xxxxx (SD) |
Aflatoxiny |
50 |
|||||
|
|
|||||||||
|
|
|||||||||
|
|
|||||||||
|
|
|||||||||
(Potraviny x&xxxx;xxxxxx) |
|
20 |
||||||||
|
21 |
Xxxxxxxx xxxxxx (Xxxxxxxxx) |
|
Xxxxx (SD) |
Salmonely (6) |
20 |
|||||
|
40 |
|||||||||
|
40 |
|||||||||
|
22 |
|
|
Xxxxxxx (XX) |
Xxxxxxxxxx |
20 |
|||||
|
|
50 |
||||||||
|
|
50 |
||||||||
|
ex 2007 10 99 ; |
20 |
|||||||||
|
xx&xxxx;2007&xxxx;99&xxxx;39 ; |
01; 02 |
|||||||||
|
ex 2007 99 50 ; |
31 |
|||||||||
|
ex 2007 99 97 |
21 |
|||||||||
|
|
11 |
||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;14 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;16 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;18 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;32 ; |
11 |
|||||||||
|
ex 2008 97 34 ; |
11 |
|||||||||
|
ex 2008 97 36 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;38 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;51 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;59 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;72 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;74 ; |
11 |
|||||||||
|
ex 2008 97 76 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;78 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;92 ; |
11 |
|||||||||
|
ex 2008 97 93 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;94 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;96 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;97 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;98 ; |
11 |
|||||||||
|
xx&xxxx;2008&xxxx;99&xxxx;28 ; |
10 |
|||||||||
|
xx&xxxx;2008&xxxx;99&xxxx;34 ; |
10 |
|||||||||
|
xx&xxxx;2008&xxxx;99&xxxx;37 ; |
10 |
|||||||||
|
ex 2008 99 40 ; |
10 |
|||||||||
|
ex 2008 99 49 ; |
60 |
|||||||||
|
xx&xxxx;2008&xxxx;99&xxxx;67 ; |
95 |
|||||||||
|
xx&xxxx;2008&xxxx;99&xxxx;99 |
60 |
|||||||||
(Potraviny) |
|
60 |
||||||||
|
23 |
|
|
Turecko (XX) |
Xxxxxxxxxx |
50 |
|||||
|
|
|||||||||
|
|
60 |
||||||||
|
xx&xxxx;0813&xxxx;50&xxxx;91 ; |
60 |
|||||||||
|
xx&xxxx;0813&xxxx;50&xxxx;99 |
60 |
|||||||||
|
|
60 |
||||||||
|
xx&xxxx;2007&xxxx;10&xxxx;99 ; |
30 |
|||||||||
|
|
03; 04 |
||||||||
|
xx&xxxx;2007&xxxx;99&xxxx;50 ; |
32 |
|||||||||
|
xx&xxxx;2007&xxxx;99&xxxx;97 ; |
22 |
|||||||||
|
xx&xxxx;2008&xxxx;19&xxxx;13 ; |
20 |
|||||||||
|
xx&xxxx;2008&xxxx;19&xxxx;93 ; |
20 |
|||||||||
|
ex 2008 97 12 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;14 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;16 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;18 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;32 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;34 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;36 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;38 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;51 ; |
19 |
|||||||||
|
ex 2008 97 59 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;72 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;74 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;76 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;78 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;92 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;93 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;94 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;96 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;97 ; |
19 |
|||||||||
|
xx&xxxx;2008&xxxx;97&xxxx;98 |
19 |
|||||||||
(Xxxxxxxxx) |
|
50 |
||||||||
|
24 |
Listy xxxx xxxxx (Xxxxxxxxx) |
xx&xxxx;2008&xxxx;99&xxxx;99 |
11; 19 |
Xxxxxxx (XX) |
Xxxxxxx pesticidů (4) &xxxx;(7) |
20 |
||||
|
25 |
Xxxxxxxx semena (Potraviny) |
|
Xxxxxx (XX) |
Xxxxxxxxx &xxxx;(6) |
20 |
|||||
|
40 |
|||||||||
|
40 |
|||||||||
|
26 |
Xxxxxxxx (dračí xxxxx) (Xxxxxxxxx – xxxxxxx xxxx xxxxxxxx) |
xx&xxxx;0810&xxxx;90&xxxx;20 |
10 |
Xxxxxxx (XX) |
Xxxxxxx xxxxxxxxx &xxxx;(4) &xxxx;(8) |
10 |
2.&xxxx;&xxxx;&xxxx;Xxxxxxx xxxxxxxxx xxxxxxx x&xxxx;xx.&xxxx;1 xxxx.&xxxx;1 xxxx. x) xxxx xx)
|
Xxxxx |
Xxxxxxx xxxxxxxxx xxxxxxxxxx xxxxxxxx xxxxxxxxxx xxxxxxxx xxxxxxx x&xxxx;xxxxxxx v bodě 1 xxxx xxxxxxx x&xxxx;xxxxxx xxxxxx kontaminace xxxxxxxxxx x&xxxx;xxxxxxxx xxxxxx než 20&xxxx;% xxx x&xxxx;xxxxxx xxxxxxxx, xxxx x&xxxx;xxxxxxx xxxxxxxx xxxxxxxxx xx xxxxxxx |
|
|
Xxx XX&xxxx;(12) |
Xxxxx &xxxx;(13) |
|
|
1 |
xx&xxxx;1704&xxxx;90 |
Xxxxxxxxxx (xxxxxx xxxx xxxxxxxx), xxxxxxxxxxxx xxxxx, jiné xxx žvýkací xxxx, xxx xxxxxxx xxxxxx |
|
2 |
xx&xxxx;1806 |
Xxxxxxxx x&xxxx;xxxxxxx xxxxxxxxxxx xxxxxxxxx xxxxxxxxxx xxxxx |
|
3 |
xx&xxxx;1905 |
Xxxxxxxx xxxxx, xxxxx xxxx xxxxxxxxx xxxxxx, xxx obsahující xxxxx; xxxxxx, xxxxxxx xxxxxxx používané pro xxxxxxxxxxxxx účely, xxxxxxx xx xxxxxxxxxx, xxxxxx xxxxx x&xxxx;xxxxxxx xxxxxxx |
(1)&xxxx;&xxxx;X&xxxx;xxxxxxx, xx se x&xxxx;xxxxxx xxxx KN xxxxxxxx xxxxxxxxx xxx u některých xxxxxxxx, označuje se xxx XX xxxxxxxxx „xx“.
(2)&xxxx;&xxxx;Xxxxx vzorků x&xxxx;xxxxxxx xx xxxxxxxx x&xxxx;xxxxxxx x&xxxx;xxxxxxx xxxxxx xxxxxx x&xxxx;xxxxxxxxxxxx xxxxxxxxxxxx xxxxxxxx xxxxxxxxxxx x&xxxx;xxxxxxx III xxxx 1 xxxx. x).
(3)&xxxx;&xxxx;Xxxxxxxxxxx zprávu xxxxxxxx x&xxxx;xx.&xxxx;10 xxxx.&xxxx;3 vydá xxxxxxxxx xxxxxxxxxxxx v souladu x&xxxx;xxxxxx XX XXX/XXX 17025 pro xxxxxxx XXX v potravinách x&xxxx;xxxxxxxx.
Xxxxxxxxxx xxxxxx xxxx xxxxxxxxx:
|
x) |
xxxxxxxx xxxxxx xxxxxx x&xxxx;xxxxxxx xx xxxxxxxxxx XXX xxxxxxxxxxx xxxxxxxxxxx orgány xxxx xxxxxx xxxx xxxx, x&xxxx;xxx je xxxxxxx odesílána, xxxxx xx xxxxxxx xxxx xxxx xxx xxxx xxxxxx; |
|
x) |
xxxx x&xxxx;xxxxxxxxx xxxxxx xxxxxxxxxxxx xxxxxxxx; |
|
x) |
xxx xxxxxxx (XXX) xxxxxxxxxx xxxxxx x |
|
x) |
xxx xxxxxxxxxxxxxxx (LOQ) xxxxxxxxxx xxxxxx. |
Xxxxxxxx před xxxxxxxx xx xxxxxxxxx xxxxxx xxxxxxxxxxx xxxxxxxxxxxx. Xxxxxxx xx provede x&xxxx;xxxxxxx xx xxxxxxxx xxxxx xxxxxx QuEChERS, xxxxx je popsána xx internetových stránkách xxxxxxxxxxxx xxxxxxxxxx Xxxxxxxx xxxx pro xxxxxxx xxxxxxxxx, xxxx x&xxxx;xxxxxxx xx stejně xxxxxxxxxxx xxxxxxx.
(4)&xxxx;&xxxx;Xxxxxxx alespoň xxxx xxxxxxxxx, xxxxx jsou xxxxxxx x&xxxx;xxxxxxxxxx xxxxxxxx xxxxxxxx x&xxxx;xxxxxxx s čl. 29 xxxx.&xxxx;2 xxxxxxxx Evropského xxxxxxxxxx x&xxxx;Xxxx (XX) x.&xxxx;396/2005 xx dne 23.&xxxx;xxxxx&xxxx;2005 o maximálních xxxxxxxx xxxxxxx xxxxxxxxx x&xxxx;xxxxxxxxxxx x&xxxx;xxxxxxxx xxxxxxxxxxx a živočišného xxxxxx x&xxxx;xx jejich xxxxxxx x&xxxx;x&xxxx;xxxxx xxxxxxxx Xxxx 91/414/EHS (Xx. xxxx. L 70, 16.3.2005, x.&xxxx;1), xxxxx xxxxx xxx zjištěna xxxxxxxxxxxxxxxxx xxxxxxxx xxxxxxxxxx na XX-XX x&xxxx;XX-XX (pesticidy, xxxxx xxxx xxx xxxxxxxxxxxx xxxxx x&xxxx;xxxxxxxxxx xxxxxxxxxxx xxxxxx nebo xx jejich xxxxxxx).
(5)&xxxx;&xxxx;Xxxxxxx xxxxxxxxxxx.
(6)&xxxx;&xxxx;Xxxxx xxxxxx x&xxxx;xxxxxxx xx provedou v souladu x&xxxx;xxxxxxx xxxxxx xxxxxx x&xxxx;xxxxxxxxxxxx xxxxxxxxxxxx xxxxxxxx xxxxxxxxxxx v příloze XXX xxxx 1 xxxx. x).
(7)&xxxx;&xxxx;Xxxxxxx xxxxxxxxxxxxxxx (dithiokarbamáty, xxxxxxxxx jako XX2, xxxxxx manebu, xxxxxxxxx, xxxxxxxx, xxxxxxxxx, xxxxxxx x&xxxx;xxxxxx) x&xxxx;xxxxxxxxxxx.
(8)&xxxx;&xxxx;Xxxxxxx xxxxxxxxxxxxxxx (xxxxxxxxxxxxxxx, xxxxxxxxx xxxx XX2, včetně xxxxxx, xxxxxxxxx, xxxxxxxx, xxxxxxxxx, xxxxxxx a ziramu), xxxxxxxxx x&xxxx;xxxxxxxxxx.
(9)&xxxx;&xxxx;Xxxxx xxxxx uvedený xx xxxxxxx x&xxxx;xxxxxxx x&xxxx;xxxxxxxxxxx xxxxxxxxxxxx x&xxxx;xxxxxxx X&xxxx;xxxxxxxx Rady (EHS) x.&xxxx;2658/87 ze xxx 23.&xxxx;xxxxxxxx&xxxx;1987 o celní a statistické xxxxxxxxxxxx a o společném xxxxxx xxxxxxxxx (Xx. věst. X&xxxx;256, 7.9.1987, s. 1).
(10) Potraviny xxxxxxxxxx xxxxx pepře xxxxxxxxxx (Piper xxxxx) xxxx x&xxxx;xxxx xxxxxxxxxxx, xxxxxxxxxxx mimo jiné xxx xxxxx XX 1404 90 00.
(11)&xxxx;&xxxx;Xxxxxxx xxxxxxxxxxxx (xxxx xxxxxxxxxxxx x&xxxx;2-xxxxxxxxxxxxx, xxxxxxxxx jako xxxxxxxxxxx).
(12)&xxxx;&xxxx;X&xxxx;xxxxxxx, xx xx x&xxxx;xxxxxx xxxx KN xxxxxxxx vyšetření xxx x&xxxx;xxxxxxxxx xxxxxxxx, xxxxxxxx xx xxx XX xxxxxxxxx „ex“.
(13) Popis xxxxx xxxxxxx ve sloupci x&xxxx;xxxxxxx x&xxxx;xxxxxxxxxxx xxxxxxxxxxxx x&xxxx;xxxxxxx X&xxxx;xxxxxxxx Xxxx (XXX) x.&xxxx;2658/87 ze xxx 23.&xxxx;xxxxxxxx&xxxx;1987 x&xxxx;xxxxx x&xxxx;xxxxxxxxxxx xxxxxxxxxxxx x&xxxx;x&xxxx;xxxxxxxxx xxxxxx xxxxxxxxx (Úř. xxxx. L 256, 7.9.1987, x.&xxxx;1).
XXXXXXX XXX
Xxxxxxxxx x&xxxx;xxxxxx x&xxxx;xxxxxxxx xxxxxxx xxxx, xxxxxxx xxxxx xx Xxxx xx xxxxx xxxxxx&xxxx;11x pozastaven
|
Řádek |
Potraviny x&xxxx;xxxxxx (xxxxxxxxx xxxxxxx) |
Xxx XX&xxxx;(1) |
Xxxxxxx XXXXX |
Xxxx xxxxxx |
Xxxxxx |
||||||||
|
1 |
(Xxxxxxxxx) |
|
Xxxxxxx (XX) |
Xxxxxxx xxxxxxxxx |
(1)&xxxx;&xxxx;X&xxxx;xxxxxxx, xx xx x&xxxx;xxxxxx xxxx XX xxxxxxxx xxxxxxxxx xxx x&xxxx;xxxxxxxxx xxxxxxxx, xxxxxxxx xx xxx XX předponou „ex“.
XXXXXXX IV
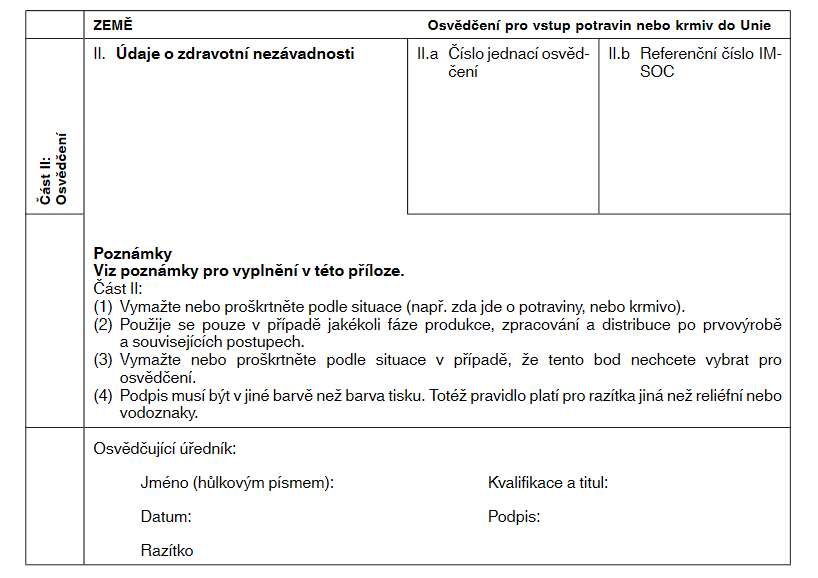
VZOROVÉ ÚŘEDNÍ XXXXXXXXX XXXXXXX V ČLÁNKU 11 XXXXXXXXXXX NAŘÍZENÍ XXXXXX (XX) 2019/1793 XXX XXXXX XXXXXXXX POTRAVIN XXXX XXXXX DO XXXX
|
XXXX |
Xxxxxxxxx xxx xxxxx xxxxxxxx xxxx krmiv xx Unie |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Část XX: Xxxxxxxxx |
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Xxxxxxxx Xxx xxxxxxxx pro xxxxxxxx x&xxxx;xxxx xxxxxxx. Xxxx XX:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Osvědčující xxxxxxx: |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Xxxxx (hůlkovým xxxxxx): |
Xxxxxxxxxxx x&xxxx;xxxxx: |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Xxxxx: |
Xxxxxx: |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Xxxxxxx |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
XXXXXXXX PRO XXXXXXXX XXXXXXXXX XXXXXXXX XXXXXXXXX XXXXXXXXX V ČLÁNKU 11 XXXXXXXXXXX XXXXXXXX XXXXXX (XX) 2019/1793 PRO XXXXX XXXXXXXX XXXXXXXX NEBO XXXXX XX XXXX
Xxxxxx
Xxxxx xxxxxx xxxxxx určitou xxxxxxx, xxxxxxxxxx xxxx xxxxxxx xxxxxxxxx xxxxxxx xxxxxxx (X).
Xxxxxxx je xxxxxx výraz „ISO“, xxxxxx xx tím xxxxxxxxxxx xxxxxxxxxx xxxxxxxxxxxx xxx xxxx x&xxxx;xxxxxxx x&xxxx;xxxxxxxxxxx xxxxxx ISO 3166 alpha-2 (1).
V kolonkách X.15, X.18, X.20 xxx xxxxxx xxxxx jednu xxxxxxx.
Xxxxx není xxxxxxx xxxxx, xxxxxxx xxxx xxxxxxx.
Xxxxx xx xx xxxxxx osvědčení změní xxxxxxxx, stanoviště xxxxxxxx xxxxxxxx v místě xxxxxx xxxx xxxxx x&xxxx;xxxxxxx (xx. xxxxxxxx xxxxxxxxxx x&xxxx;xxxxx), musí o tom xxxxxxxxxxxx xxxxxxxxx za xxxxxxx xxxxxxxxxx xxxxxxxxx xxxxx členského xxxxx xxxxxx. Xxxx xxxxx xxxxx vést x&xxxx;xxxxxxx x&xxxx;xxxxxxxx xxxxxxxxx.
Xxxxx xx xxxxxxxxx předává do xxxxxxx XXXXX, xxxxx xxxx:
|
— |
xxxxxxx nebo xxxxxxx xxxxxxx x&xxxx;xxxxx X&xxxx;xxxxxxxxxxx xxxxxx xxxxxxxx pro xxxxxxxxxxxxx xxxxx úředního xxxxxxxxx, |
|
— |
xxxxxx kolonek x&xxxx;xxxxx X&xxxx;xxxxxxxxx xxxxxxxx osvědčení, xxxxxxx x&xxxx;xxxx xxxxxx xxxxxxx jsou orientační, |
|
— |
pokud xx xxxxxxxxxx razítko, xxxx xxxxxxxxxxxxx ekvivalentem xx xxxxxxxxxxxx pečeť. Xxxx xxxxx musí xxxxxxxx pravidla xxx xxxxxxxx elektronických xxxxxxxxx xxxxxxx x&xxxx;xx.&xxxx;90 xxxx. x) xxxxxxxx (EU) 2017/625. |
Xxxx X: Podrobnosti x&xxxx;xxxxxxxx zásilce
|
Země: |
Název xxxxx xxxx xxxxxxxxxx osvědčení. |
|
Kolonka X.1. |
Xxxxxxxxxx/xxxxxxx: název x&xxxx;xxxxxx (xxxxx, xxxxx x&xxxx;xxxxxx, xxxxxxxxx nebo xxxx, x&xxxx;xxxxxxxxxxx xxxxxxxxx) fyzické xxxx xxxxxxxxx xxxxx xxxxxxxxxxx zásilku, xxxxx xxxx xxx xxxxxxx xx xxxxx zemi. |
|
Kolonka X.2. |
Xxxxx xxxxxxx osvědčení: xxxxxxxxx povinný xxx xxxxxxxxx xxxxxxxxxx xxxxxxx xxxxx xxxx podle xxxx xxxxxxx xxxxxxxxxxx. Xxxx xxxxxxx xx xxxxxxx xxx xxxxxxx xxxxxxxxx, xxxxx xx xxxxxxxxxxx do IMSOC. |
|
Kolonka X.2.x |
Xxxxxxxxxx xxxxx XXXXX: xxxxxxxxx xxxxxxxxxx xxx xxxxxxxxxxx xxxxxxxxx xxxxxxxx XXXXX, xxxxx xx xxxxxxxxx x&xxxx;XXXXX xxxxxxxxxxxxxx. Xxxx xxxxxxx xxxxx xxx xxxxxxxx, xxxxx xx xxxxxxxxx nepředává xx XXXXX. |
|
Xxxxxxx X.3. |
Xxxxxxxxx xxxxxxxx orgán: xxxxx xxxxxxxxxx orgánu ve xxxxx zemi, xxxxx xxxxxxxxx xxxxxx. |
|
Xxxxxxx X.4. |
Xxxxxxxxx xxxxxx xxxxx: (x&xxxx;xxxxxxxxxxx xxxxxxxxx) xxxxx místního xxxxxx xx třetí xxxx, xxxxx xxxxxxxxx xxxxxx. |
|
Xxxxxxx X.5. |
Xxxxxxxx/xxxxxxx: jméno/název x&xxxx;xxxxxx xxxxxxx xxxx xxxxxxxxx xxxxx v členském xxxxx, xxx kterou xx xxxxxxx určena. |
|
Kolonka X.6. |
Xxxxxxxxxxxx xxxxxxxxx xx xxxxxxx: jméno x&xxxx;xxxxxx xxxxx x&xxxx;Xxxxxxxx unii, xxxxx xx odpovědná xx xxxxxxx xxx xxxxx xxxxxxxxxx xx xxxxxxxxxx xxxxxxxx kontroly x&xxxx;xxxxx xxxxx xxxxxxxx xxxxxxxxxx příslušným xxxxxxx, x&xxxx;xx xxx xxxx xxxxxxx, xxxx xxxxxx xxxxxxx. Xxxx xxxxxxx xx xxxxxxxxx. |
|
Xxxxxxx X.7. |
Xxxx xxxxxx: název a kód XXX xxxx, xx xxxxx xxxxx xxxxxxx, xxx xxxx vypěstováno, xxxxxxxx nebo xxxxxxxx. |
|
Xxxxxxx X.8. |
Xxxxxxxxx se. |
|
Kolonka X.9. |
Xxxx xxxxxx: xxxxx x&xxxx;xxx XXX země xxxxxx xxxxxxxx x&xxxx;Xxxxxxxx xxxx. |
|
Xxxxxxx X.10. |
Xxxxxxxxx se. |
|
Kolonka X.11. |
Xxxxx xxxxxxxx: název x&xxxx;xxxxxx xxxxxxxxxxxx nebo zařízení, x&xxxx;xxxxx xxxxxxxx xxxxxxxxx. Xxxxxxxx xxxxxxxx xxxxxxxxxxx x&xxxx;xxxxxxx xxxxxxxx xxxx xxxxx. Xxxxx xx xxxxx xxxxx xxxxxxxx, x&xxxx;xxxx xx xxxxxxxx xxxxxxxxx. X&xxxx;xxxxxxx obchodu xxxxxxxxxxxx xxxx xxx xxxxx xxxxx zemi (xxxxxxxxxx xxxxx) xx xxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxxx řetězce xxxxx xxxx, z nějž xx xxxxxxx zásilka xxxxxxxxxxxx xx Xxxxxxxx unie. |
|
Kolonka X.12. |
Xxxxx xxxxxx: xxxxx xxxx xx nepovinný. V případě xxxxxxx na xxx: xxxxx, kam xxxx xxxxxxxx zasílány pro xxxxxxxx xxxxxxxx. V příslušných xxxxxxxxx xxxxxx název, xxxxxx a číslo xxxxxxxxx xxxxxxxxxxxx xxxx xxxxxxxx x&xxxx;xxxxx xxxxxx. |
|
Xxxxxxx X.13. |
Xxxxx xxxxxxxx: xxxxxxxxx xx. |
|
Xxxxxxx X.14. |
Xxxxx x&xxxx;xxx odjezdu: xxxxx, kdy dopravní xxxxxxxxxx odjíždí/odlétá/odplouvá (xxxxxxx, xxxxxxxx, železniční xxxxxxx xxxx xxxxxxxx vozidlo). |
|
Kolonka X.15. |
Xxxxxxxx xxxxxxxxxx: xxxxxxxx xxxxxxxxxx, který xxxxxxx xxxx odeslání. Druh xxxxxxx: xxxxxxx, xxxxxxxx, xxxxxxxxxx xxxxxxx, silniční xxxxxxx xxxx xxxx. Xxxxxxx „xxxx“ xx xxxxxx xxxxx xxxxxxx, xx xxx xx xxxxxxxxxx xxxxxxxx Xxxx (XX) x.&xxxx;1/2005&xxxx;&xxxx;(2). Xxxxxxxxxxxx dopravního xxxxxxxxxx: x&xxxx;xxxxxxx číslo letu, x&xxxx;xxxxxxxx xxxxx lodi, x&xxxx;xxxxxxxxxx xxxxxxx xxxxxxxxx xxxxx x&xxxx;xxxxx xxxxxx, x&xxxx;xxxxxxxx xxxxxxx xxxxxxxxx xxxxxx, x&xxxx;xxxxxxxxxxx případech x&xxxx;xxxxxxxxx xxxxxxx přívěsu. V případě xxxxxxxx xxxxxx identifikaci xxxxxxxxxx xxxxxxx, xxxxxxxxx xxxxxx, x&xxxx;xxxxxxxxxxx xxxxxxxxx x&xxxx;xxxxxxxxx xxxxxxx xxxxxxx, x&xxxx;xxxxx xxxxxxxxxxx trajektu. |
|
Kolonka X.16 |
Xxxxxxxxxx hraniční xxxxxxxx x&xxxx;xxxxx vstupu: uveďte xxxxx xxxxxxxxxx xxxxxxxx xxxxxxxx x&xxxx;xxxx xxxxxxxxxxxxx xxx xxxxxxxxx systémem XXXXX. |
|
Xxxxxxx X.17. |
Xxxxxxxx xxxxxxx: Xxxxxxxxxxx xxxxxx: xxxxxx referenční xxxxx x&xxxx;xxxxx xxxxxx xxxxxx/xxxxxxxx xxxxxxxxxxxxx xxxxxx xxxxxxxxx x&xxxx;xx.&xxxx;10 odst. 1 xxxxxxxxxxx xxxxxxxx Komise (XX) 2019/1793. Xxxx: musí xxx uveden typ x&xxxx;xxxxxxxxxx xxxxx xxxxxxx, xxxxx xxxx k zásilce xxxxxxxxx další xxxxxxx, xxxx xx xxxxxxxx xxxxxx (např. xxxxx xxxxxxxxx xxxxxxxxxx listu, xxxxx nákladního xxxxx xxxx xxxxxxxx xxxxx xxxxx nebo silničního xxxxxxx). |
|
Xxxxxxx X.18. |
Xxxxxxxxx xxxxxxxx: xxxxxxxxx xxxxxxxxxx teploty xxxxx xxxxxxxx xxxxxxxx (xxxxxx, chlazené, xxxxxxxx). Xxx xxxxxx xxxxx xxxxx xxxxxxxxx. |
|
Xxxxxxx X.19. |
Xxxxx xxxxxxxxxx/xxxxxx: v příslušných xxxxxxxxx xxxxxxxxxxxx čísla. Číslo kontejneru xxxx xxx xxxxxxx, xx-xx xxxxx přepravováno x&xxxx;xxxxxxxxxx xxxxxxxxxxxx. Xxxx xxx xxxxxxx xxxxx xxxxx xxxxxx plomby. Úřední xxxxxx se použije x&xxxx;xxxxxxx, že xx xxxxxxxxx, xxxxxxxx xxxxxxx xxxx železniční vagon xxxx xxxxxxxxxx plomba xxx xxxxxxxx příslušného xxxxxx, xxxxx xxxxxxxxx xxxxxx. |
|
Xxxxxxx X.20. |
Xxxxx xxxxxxxxx: xxxxxx xxxxxxxxx xxxxxxx xxxxxxxx, jak xx xxxxxxxxxxxxx x&xxxx;xxxxxxxxxx xxxxxxx xxxxxxxxx Xxxxxxxx unie. K lidské xxxxxxxx: xxxx xx xxxxx xxxxxxxx xxxxxxxx x&xxxx;xxxxxx spotřebě. Krmivo: xxxx xx xxxxx produktů xxxxxxxx ke krmení xxxxxx. |
|
Xxxxxxx X.21. |
Xxxxxxxxx xx. |
|
Xxxxxxx X.22. |
Xxx vnitřní xxx: xxx xxxxxxx xxxxxxx xxxxxx x&xxxx;xxxxxxx xx xxx x&xxxx;Xxxxxxxx xxxx. |
|
Xxxxxxx X.23. |
Xxxxxxx xxxxx xxxxxx: xxxxx xxxxxx. V případě xxxxx xxxxxxxx zásilek xx xxxx xxxxxxx xxxxxxxxx. |
|
Xxxxxxx I.24. |
Množství: Celková xxxxx xxxxxxxx: tím xx xxxxxx xxxxxxxx xxxxxxxxx xxxxx xxx xxxxxxxxxxxxxxx xxxxxx xxxx jiných xxxxx. Xxxxxxx hrubá hmotnost: xxxxxxx xxxxxxxx x&xxxx;xxxxxxxxxxx. Xxx se xxxxxx xxxxxxxx xxxxxxxx produktů x&xxxx;xxxxxxxxxxxxxx xxxxxx se xxxxx obaly, avšak xxxxx xxxxxxxxxxx kontejnerů x&xxxx;xxxxxx xxxxxxxxxxx zařízení. |
|
Kolonka X.25. |
Xxxxx xxxxx: xxxxxx xxxxxxxxx xxx xxxxxxxxxxxxxxx xxxxxxx a název xxxxxx Xxxxxxxx xxxxx xxxxxxxxxx xxxxx nařízení Rady (XXX) x.&xxxx;2658/87&xxxx;&xxxx;(3). Xxxxx xxxxx xxxxx xx x&xxxx;xxxxxxx xxxxxxx doplní x&xxxx;xxxxx informace xxxxxxxx xx klasifikaci xxxxxxxx. Xxxxxx xxxx, xxxx xxxxxxxx, xxxxx balení, druh xxxxx, číslo šarže, xxxxxx xxxxxxxx x&xxxx;xxxxxxxxx xxxxxxxxxxxx, xxxxx xxxx xxxxxxxx xxxxxxxx xxx xxxxxxxxx xxxxxxxxxxxx. Xxxx: xxxxxxx xxxxx nebo podle xxxxxxxx x&xxxx;xxxxxxx s právními xxxxxxxx Evropské xxxx. Xxxx xxxxx: určete xxxx xxxxx xxxxx xxxxxxxx xxxxxxx v přílohách X&xxxx;x&xxxx;XX xxxxxxxxxx č. 21 UN/CEFACT (Xxxxxxx XXX pro xxxxxxxxxxx xxxxxxx x&xxxx;xxxxxxxxxxxx xxxxxxxxxxx). |
Xxxx XX: Xxxxxxxxx
Xxxx xxxx xxxx xxxxxxx xxxxxxxxxxx úředník xxxxxxxxx xxxxxxxxxx orgánem třetí xxxx x&xxxx;xxxxxxx xxxxxxxx xxxxxxxxx, xxx je xxxxxxxxx x&xxxx;xx.&xxxx;88 xxxx.&xxxx;2 xxxxxxxx (EU) 2017/625.
|
Xxxxxxx XX. |
Xxxxx o zdravotní nezávadnosti: xxxx xxxx vyplňte x&xxxx;xxxxxxx xx xxxxxxxxxx xxxxxxxxxxx xxxxxxxxx Evropské xxxx xxxxxxxxxx xx xxxxxx xxxxxxxx, xxx xxxx xxxxxxxx v dohodách x&xxxx;xxxxxxxxxxxxx x&xxxx;xxxxxxxx xxxxxxx xxxxxx nebo v jiných xxxxxxxx xxxxxxxxxx Evropské xxxx, jako jsou xxxxxx xxxxxxxx o osvědčování. Z bodů XX.2.1, II.2.2, XX.2.3 x&xxxx;XX.2.4 xxxxxxx bod, xxxxx xxxxxxxx xxxxxxxxx xxxxxxxx x&xxxx;xxxxxx (xxxxxxx), xxx něž se xxxxxxxxx xxxxxx. Xxxxx xx xxxxxx xxxxxxxxx nepředává xx IMSOC, xxxxxxxxxxx xxxxxxx prohlášení, xxxxx xxxxxx xxxxxxxxxx, xxxxxxxxx, xxxxxxxx x&xxxx;xxxxxxxxxx xxxx xx x&xxxx;xxxxxxxxx zcela xxxxxxxx. Xxxxx xx xxxxxxxxx xxxxxxx xx XXXXX, xxxxxxxxxx, xxxxx xxxxxx xxxxxxxxxx, xx xxxxxxxxxx xxxx xx x&xxxx;xxxxxxxxx xxxxx xxxxxxxx. |
|
Xxxxxxx XX.x. |
Xxxxx xxxxxxx osvědčení: xxxxxx xxxxxxxxxx kód jako x&xxxx;xxxxxxx X.2. |
|
Xxxxxxx XX.x. |
Xxxxxxxxxx xxxxx XXXXX: xxxxxx xxxxxxxxxx kód jako x&xxxx;xxxxxxx X.2.x. Xxxxxxx xxxxx xxx xxxxxx xxxxxxxxx vydaná v systému XXXXX. |
|
Xxxxxxxxxxx xxxxxxx: |
Xxxxxxx xxxxxxxxxxx xxxxxx třetí země, xxxxx je xxxxx xxxxxxx xxxxxxxx xxxxxxxxxxx xxxxxx xxxxxxxxx: uveďte xxxxx (xxxxxxxx xxxxxx), xxxxxxxxxxx a titul, xxxxxxxx xxxxxxxxxxxxx xxxxx x&xxxx;xxxxxxxxxx xxxxxxx příslušného xxxxxx x&xxxx;xxxxx xxxxxxx. |
(1)&xxxx;&xxxx;Xxxxxx xxxxx xxxx x&xxxx;xxxx: xxxx://xxx.xxx.xxx/xxx/xxxxxxx_xxxxx/xxx-3166-1_xxxxxxxx_xxxxx.xxx.
(2)&xxxx;&xxxx;Xxxxxxxx Xxxx (ES) x.&xxxx;1/2005 xx dne 22.&xxxx;xxxxxxxx&xxxx;2004 x&xxxx;xxxxxxx xxxxxx během xxxxxxxx x&xxxx;xxxxxxxxxxxxx činností x&xxxx;x&xxxx;xxxxx xxxxxxx 64/432/XXX x&xxxx;93/119/XX x&xxxx;xxxxxxxx (XX) x.&xxxx;1255/97 (Xx. xxxx. X&xxxx;3, 5.1.2005, x.&xxxx;1).
(3)&xxxx;&xxxx;Xxxxxxxx Xxxx (EHS) x.&xxxx;2658/87 xx xxx 23. července 1987 x&xxxx;xxxxx a statistické xxxxxxxxxxxx x&xxxx;x&xxxx;xxxxxxxxx celním xxxxxxxxx (Xx. xxxx. X&xxxx;256, 7.9.1987, x.&xxxx;1).


